siRNA in Gastrointestinal Disorders-Digesting the Possibilities
Introduction to siRNA in Gastrointestinal Disorders
Research interest in siRNA therapy has developed and widened throughout recent decades. Since the discovery of RNA interference (RNAi) in 1998 scientists have developed synthetic oligonucleotides which target specific genes to silence them thereby expanding disease treatment possibilities. The RNAi pathway functions to enhance mRNA degradation which leads to reduced translation of unwanted sequences. The application of siRNA to silence gene expression holds promise for treating many acute and chronic conditions affecting the gastrointestinal system. Research conducted in laboratory settings has proven siRNA effectiveness across various disease models while ongoing animal studies continue to generate supporting in vivo data. This technology shows a major benefit because it allows genomic data to be quickly transformed into effective treatments. The potential of RNAi as a pharmaceutical development candidate rises when it demonstrates persistent gene silencing with no systemic side-effects.
Introduction to Gastrointestinal Disorders
- Overview different Gastrointestinal Disorders
The gastrointestinal tract lining suffers from several common diseases such as inflammatory bowel disease (IBD), celiac disease, malignancy and gastroesophageal reflux disease together with eosinophilic esophagitis and additional medical conditions. IBD involves chronic inflammatory disorders like Crohn's disease and ulcerative colitis which produce ongoing inflammatory episodes across the entire gastrointestinal system. IBD develops through complicated interplay between genetic components and environmental factors which trigger immune responses that cause abdominal pain and gastrointestinal symptoms including weight loss and diarrhea. Patients with gastrointestinal cancers including colorectal and gastric cancer face poor prognoses and high mortality rates because these cancers form due to genetic mutations combined with long-term inflammation. Immunosuppressive medications together with biological agents that block pro-inflammatory cytokines such as TNF-α form the treatment strategy for inflammatory bowel diseases. A portion of patients benefit from these treatments but experience serious side effects along with reduced effectiveness as time passes. The management of gastrointestinal cancer involves surgical procedures and chemotherapy along with targeted therapies but achieving continuous remission proves difficult because treatment-related toxicity remains hard to control.
- siRNA as a Therapeutic Approach
siRNA acts as a precise therapeutic instrument by turning off specific genes which play a role in inflammation and cancer formation. siRNA treatments that focus on TNF-α show promise in reducing inflammation in IBD experimental models. Targeted siRNA treatments designed to silence oncogenes or tumor suppressor genes could improve treatment outcomes for gastrointestinal cancer patients. The siRNA consists of a nucleotide sequence with 19-23 base pairs and moves to the cytoplasm where it binds with the RNA-induced silencing complex (RISC). The sense strand gets degraded after binding to RISC while the antisense strand remains as part of RISC. The RISC executes multiple mRNA cleavages that suppress the expression of a single gene. By focusing its action on single gene expression this therapeutic approach shows potential as an alternative to existing treatments which suppress the immune system broadly or harm non-targeted organs and provoke immune responses.
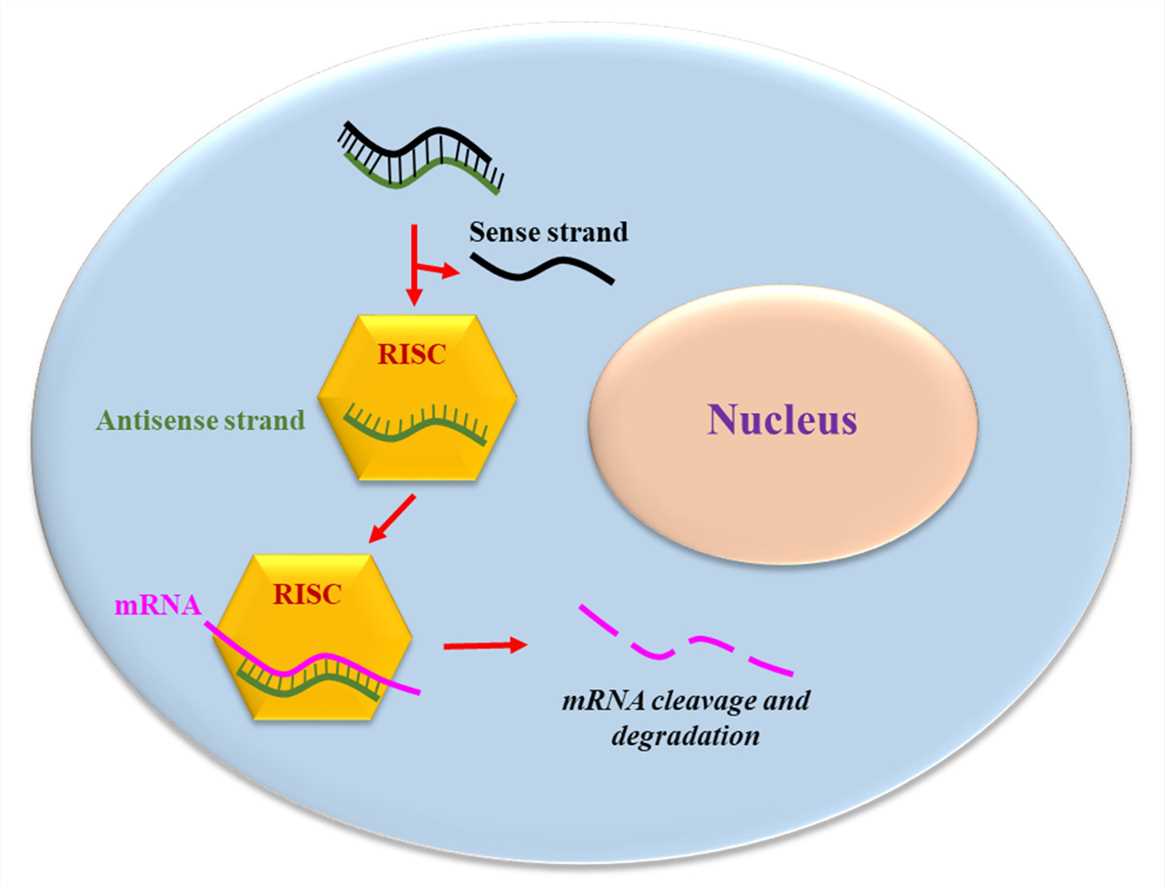 Fig.1 The siRNA mechanism of action1,6.
Fig.1 The siRNA mechanism of action1,6.
siRNA in Gastrointestinal Biology
- Targeting Genes Involved in Gut Inflammation
IBD manifests as persistent gastrointestinal inflammation which stems from abnormal immune system activity. TNF-α and IL-6 cytokines function as crucial elements in developing IBD through their involvement in disease progression. The pro-inflammatory cytokine TNF-α drives inflammation and tissue damage whereas IL-6 orchestrates immune responses and facilitates Th17 cell differentiation. In IBD patients cytokine levels become elevated and lead to disease symptoms including abdominal pain and diarrhea as well as weight loss. The process of designing siRNA enables it to bind to the mRNA of specific cytokines and their receptors which decreases their expression levels and helps control inflammation. Researchers employed gemini lipid nanoparticles (GLNPs) to introduce TNF-α siRNA into colon epithelial cells and macrophages along with dendritic cells. The use of GLNPs led to reduced TNF-α expression and alleviated gut inflammation in mice colonized with DSS-induced colitis. A research team created glucan-encapsulated siRNA particles (GeRPs) that target and deliver siRNA to Map4k4 kinase which plays a role in TNF-α signaling. The GeRPs caused a significant decrease in TNF-α and IL-1β levels in mice which shows that siRNA holds promise for IBD treatment.
- Inhibition of Oncogenes in Colorectal Cancer
Mutations in oncogenes like KRAS and c-Myc drive colorectal cancer which remains a major cause of cancer-related deaths. siRNA targets oncogenes to silence their activity which leads to reduced tumor growth and metastasis. Researchers used lipofectamine for siRNA delivery against AEG-1 which functions as an oncogene that supports cell proliferation and survival. The siRNA treatment significantly reduced AEG-1 expression levels while simultaneously inhibiting cell proliferation in gastric cancer cell lines. Preclinical models indicate that oncogene silencing through siRNA administration effectively diminishes both tumor expansion and metastatic spread. Researchers utilized attenuated Salmonella to transport siRNA aimed at PD-1 which serves as an immunosuppressive receptor playing a key role in tumor immune escape. The procedure demonstrated effective PD-1 expression reduction while causing cancer cell apoptosis and limiting tumor cell migration in a colorectal cancer mouse model. Research demonstrated that siRNA targeting KRAS in colorectal cancer cells resulted in decreased tumor growth and metastasis.
Delivery methods for siRNA-based drugs in preclinical studies
- Oral delivery systems
Oral therapies stand out as the preferred delivery method because they offer straightforward administration alongside better safety profiles and enhanced patient compliance. In 2009 researchers introduced oral siRNA delivery for the treatment of IBD. The creation of 1,3-d-glucan shells from baker's yeast was performed through a solvent extraction method. The shells contained unmodified siRNA molecules sandwiched between polyethylenimine (PEI) polymer layers while researchers named the product glucan-encapsulated siRNA particles (GeRPs). SiRNA targeting Map4k4, which regulates TNF-α signaling at germinal centers, was loaded into GeRPs. The glucan component in GeRPs specifically targets the M cells located in intestinal Peyer's patches which are specialized cells in the intestinal epithelium responsible for moving antigens from the GI tract lumen to the immune system. M cells ingest GeRPs through the beta 1,3-d-glucan receptor pathway and following internalization, phagosomes with acidic pH conditions permit siRNA to leak through the glucan particle's porous outer wall. Mice that received an oral gavage of Map4k4 siRNA with GeRPs experienced an 80% reduction in TNF-α and IL-1β protein expression compared to those given scrambled siRNA. The lethality linked to LPS injection dropped when using GeRPs with either Map4k4 siRNA or one of two alternative TNF siRNA types.
- Rectal Delivery Systems
The intestine can receive topical therapy through rectal administration. Patients with ulcerative colitis that affects only the rectum may receive full rectal formulation treatments for total coverage. Researchers expanded upon their previous achievements in enhancing transdermal permeability through ultrasound by examining how this technique could boost siRNA delivery effectiveness in mouse colon tissue. Researchers delivered TNF-α siRNA in water rectally before applying two ultrasound pulses at 40 kHz through an internal probe. The ultrasound treatment resulted in TNF-α silencing efficiency increasing 7–8 times over controls in mice. Researchers administered rectal doses of all 3 siRNA Calcium phosphate (Ca-P) nanoparticles to DSS colitis mice between days 2 and 5 after colitis induction. Nanoparticles were absorbed more quickly by the inflamed colon than by the uninflamed colon mainly within intestinal epithelial cells and mesenteric lymph nodes. The colon showed a 40% decrease in TNF-α mRNA expression levels and reduced keratinocide-derived cytokine and IP-10 expression by up to 50%.
- Esophageal Delivery Systems
The esophagus represents the uppermost section of the GI tract and serves to move food while not participating in digestion or nutrient absorption. Esophageal pathology typically results from inflammatory diseases as well as cancer or physical injury. Esophageal stricturing develops as a common consequence of organic disease or medical procedures which results in dysphagia and odynophagia as well as food becoming stuck. The medical research community has explored how delivering siRNA directly to the esophageal region can help reduce the impact of strictures. Scientists applied carbohydrate sulfotransferase 15 (CHST15) siRNA to prevent complications that occur with endoscopic submucosal dissection which is used to remove esophageal carcinoma. The procedure's extensive tissue manipulation leads to common postoperative inflammation that results in mucosal contraction. The transmembrane Golgi protein CHST15 plays a role in fibrosis development during mouse colitis and myocarditis. The CHST15 enzyme generates sulfated disaccharide units of chondroitin sulfate for the extracellular matrix and likely plays a role in post-resection fibrosis development. Researchers conducted a semicircular endoscopic submucosal dissection on juvenile pigs and then applied CHST15 siRNA as a single injection into the ulcer that formed. The endoscopic evaluation on day 7 revealed that the esophagus displayed reduced strictures and demonstrated significantly diminished mucosal contraction.
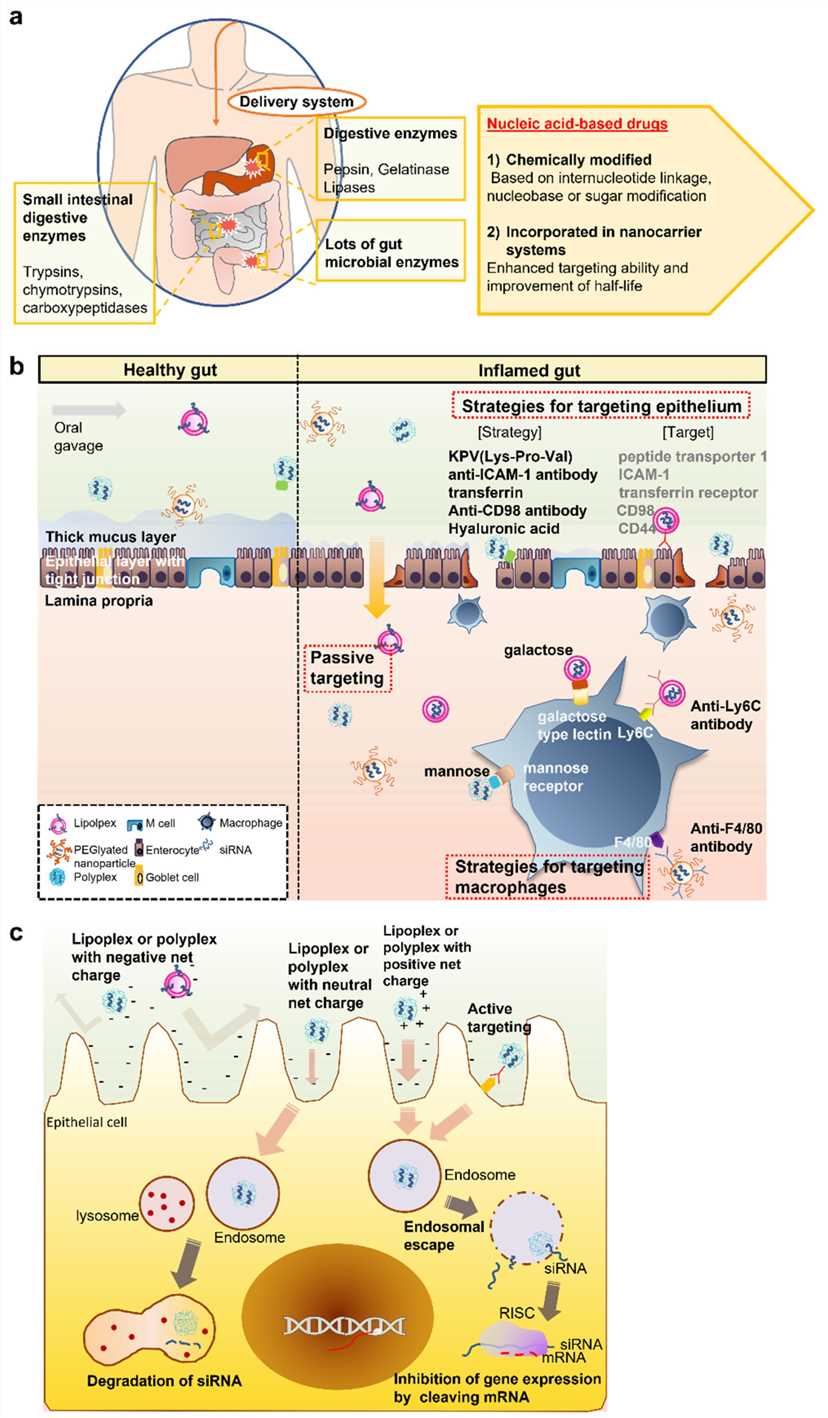 Fig. 2 Physiological and biological barriers and strategies to orally deliver siRNA drugs2,6.
Fig. 2 Physiological and biological barriers and strategies to orally deliver siRNA drugs2,6.
siRNA Chemical Modifications in preclinical models for Gastrointestinal Research
The research team examined different siRNA modifications including 2′-O-methylation, LNAs, phosphorothioate linkages and propanediol modification at the 3′ end to determine their effectiveness in silencing TNF-α mRNA production within murine peritoneal exudate macrophages. The combination of 3′-end propanediol modification and dual methylation at the 5′-end of TNF-α siRNA (siTNF-OMe-P) resulted in the highest silencing effectiveness for TNF-α compared to both unmodified TNF-α siRNA and other chemical modifications tested. During the fetal bovine serum degradation assay siTNF-OMe-P maintained superior stability compared to all other samples after 24 hours. Two doses of siTNF-OMe-P suspended in media were administered to DSS colitis mice within a period of 4 days. The mice receiving siTNF-OMe-P solution demonstrated TNF-α protein levels comparable to healthy mice while showing improved colon appearance alongside reduced myeloperoxidase (MPO) levels. Colon tissue treated with siTNF-OMe-P showed higher expression of tissue repair genes Claudin-7 and ssh2 when compared to controls while maintaining expression levels similar to healthy epithelium genes. Researchers exposed peripheral blood mononuclear cell cultures to different siRNA modifications to determine immune response. The modified siRNA conditions reduced h-TNF-α levels which serve as a TLR activation marker and are directly blocked by TNF-α siRNA in vitro.
Future directions in siRNA-based therapies
The field of oral nanomedicines has made progress during the last decade yet several critical issues need resolution before clinical translation can succeed. The efficiency of intracytosolic siRNA delivery needs improvement as a primary focus. For siRNA drugs to function therapeutically they need to leave endosomes through mechanisms like proton sponge effects and osmotic lysis. Most developed nanomedicines show poor endosomal escape efficiency which means additional research is needed to enhance this performance. The present lipoid nanoparticles (LNPs) stand out as the most effective delivery systems for siRNA drugs in clinical practice but still face issues of limited endosomal escape. Efficient delivery into the cytosol can be achieved through the use of pH-responsive lipid or polymer-based lipoplexes or polyplexes. In addition, complexity issues should be addressed. The complex structures of oral nanomedicines delivering siRNA drugs create significant hurdles for overcoming the previously discussed barriers. The challenges of mass-producing nanoparticles and maintaining quality control present obstacles that threaten successful clinical translation. The production cost increases when multiple components are involved which requires consideration. siRNA-mediated therapeutics show effectiveness in managing intestinal barrier disruption and immune system dysregulation in IBD treatment but the beneficial manipulation of the dysbiotic gut microbiome remains important.
References
- Losurdo, Pasquale, et al. "Potential application of small interfering RNA in gastro-intestinal tumors." Pharmaceuticals 15.10 (2022): 1295. https://doi.org/10.3390/ph15101295.
- Shinn, Jongyoon, et al. "Oral nanomedicines for siRNA delivery to treat inflammatory bowel disease." Pharmaceutics 14.9 (2022): 1969. https://doi.org/10.3390/pharmaceutics14091969.
- Gabel, Michael, et al. "Surface design options in polymer-and lipid-based siRNA nanoparticles using antibodies." International Journal of Molecular Sciences 23.22 (2022): 13929. https://doi.org/10.3390/ijms232213929.
- Liu, Ping, et al. "Oxidative stress and antioxidant nanotherapeutic approaches for inflammatory bowel disease." Biomedicines 10.1 (2021): 85. https://doi.org/10.3390/biomedicines10010085.
- Zhang, Wunan, Cecilia Bohns Michalowski, and Ana Beloqui. "Oral delivery of biologics in inflammatory bowel disease treatment." Frontiers in Bioengineering and Biotechnology 9 (2021): 675194. https://doi.org/10.3389/fbioe.2021.675194.
- Distributed under Open Access license CC BY 4.0, without modification.
