Selection and Optimization of Gene Therapy Vectors
What is Gene Therapy
Gene therapy represents an innovative medical treatment method which shows great promise for addressing numerous illnesses by addressing their genetic origins. Gene therapy functions by delivering GM (GM) into patient cells to address defective genes. Researchers use multiple gene therapy techniques including mutation repair, gene replacement, and new gene introduction to boost disease resistance in patients. In genetic disorders such as cystic fibrosis or hemophilia gene therapy works by delivering functional copies of defective genes directly to patient cells as a method to treat the disease's root cause. Traditional treatments differ greatly from gene therapy because they focus on symptom management rather than tackling the genetic root of disorders. Through its ability to provide prolonged and potentially permanent remedies gene therapy stands out as an exceptional area of advancement in contemporary medical science.
Importance of Vectors in Gene Therapy
Vectors serve a fundamental function in gene therapy success by transporting therapeutic GM to target cells. The absence of efficient and safe vectors would prevent therapeutic genes from reaching their target destinations inside the body which makes the entire gene therapy process futile. Vectors need to accomplish two essential tasks: they must transfer GM into target cells and ensure that this material is correctly expressed. Delivery vectors must navigate several biological obstacles including immune defenses as well as cellular membranes and internal compartments such as the nucleus. Vectors should be engineered to reduce potential hazards which include provoking immune reactions and creating unwanted genetic changes. Choosing the right vector for gene therapy determines its success rate because it affects how effective and safe the treatment is. An essential step in the development of effective gene therapies involves selecting and optimizing the vector which matches specific therapeutic needs.
What is the Purpose of Gene Therapy?
Gene therapy functions to improve treatment outcomes and develop potential genetic condition cures through direct targeting of core genetic mutations. Unlike treatments that focus solely on managing symptoms gene therapy aims to correct genetic defects which enables normal bodily function. Gene therapy for monogenic disorders including Duchenne muscular dystrophy involves delivering a functional dystrophin gene to achieve normal muscle function. Scientists recognize the opportunities to use gene therapy for acquired diseases in addition to its use for genetic disorders. An application of gene therapy includes strengthening immune defenses against cancer through the delivery of genes that activate immune cells. Gene therapy provides a preventive measure for high-risk individuals by addressing potential genetic disease onset based on their genetic predispositions. Gene therapy functions as a proactive disease management strategy by fixing or replacing faulty genes before any symptoms develop. Gene therapy aims to deliver enduring medical advantages that enhance patient quality of life.
Basic Types of Vectors
- Viral Vectors
Vectors used in gene therapy can be broadly categorized into two main types: viral vectors and non-viral vectors. Scientists genetically modify viruses to create viral vectors suitable for human applications. Because these viruses can naturally infect cells and transfer GM, they become powerful tools for gene therapy applications. The most common viral vectors used in gene therapy treatments are adenoviruses together with adeno-associated viruses (AAV) and lentiviruses. Each viral vector shows distinct characteristics together with particular advantages when used. AAV vectors demonstrate low immune response levels and deliver sustained gene expression which qualifies them for widespread therapeutic use.
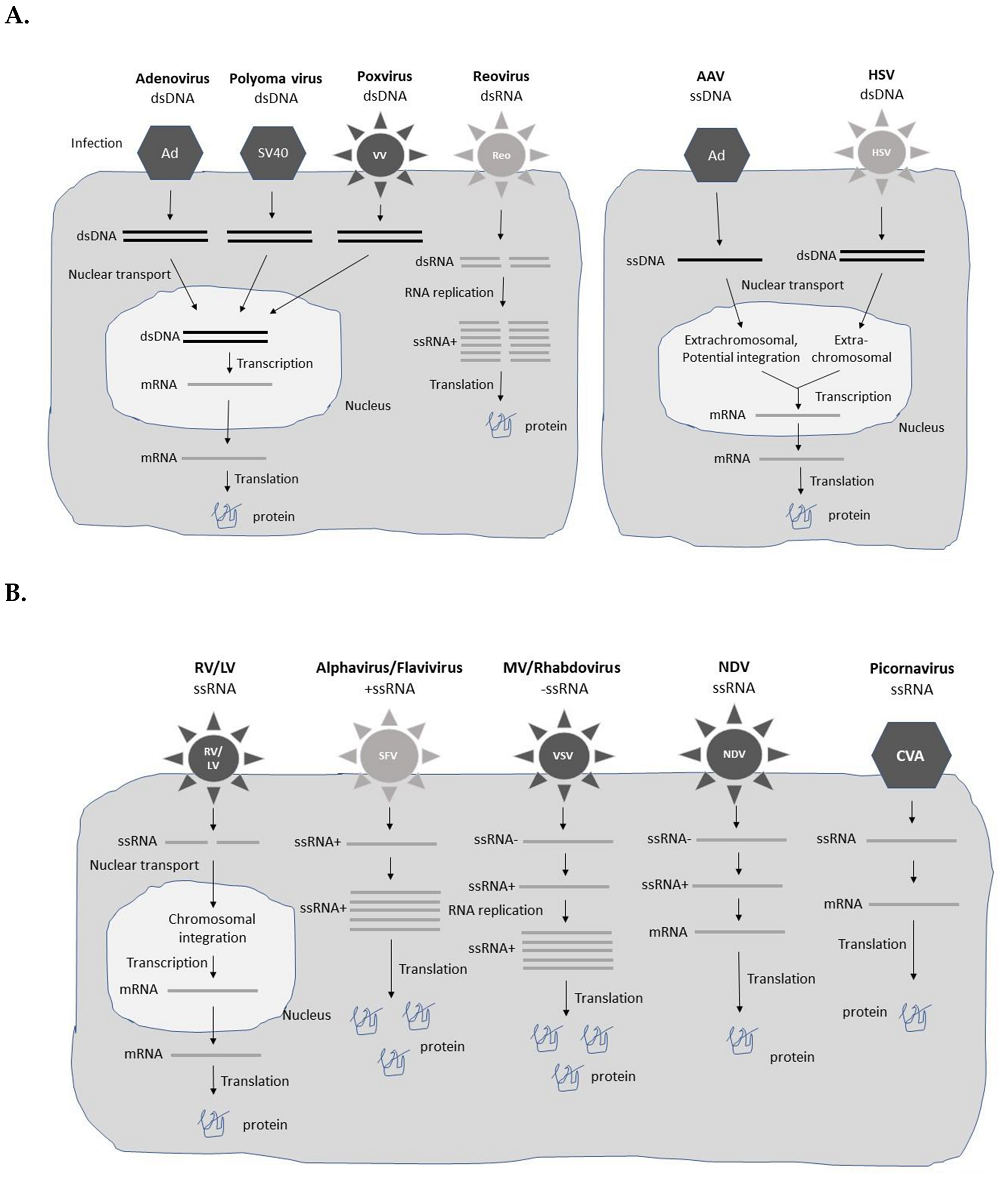 Fig.1 Viral vector expression systems1,7.
Fig.1 Viral vector expression systems1,7.
- Non-viral Vectors
A range of non-viral vectors exists such as plasmid DNA, nanoparticles, and liposomes along with other non-viral delivery systems. Physical and chemical methods enable these vectors to transfer GM into cells. Non-viral vectors avoid using viruses to serve as their fundamental delivery component. These gene delivery systems use alternative methods to transport GM into cells. Nanoparticles provide protection for DNA while enabling its delivery to target cells through multiple administration routes. Small vesicles created from lipid bilayers called liposomes function as carriers to package GM for delivery purposes. Non-viral vectors are deemed safer options since they avoid the viral infection risks and potential insertional mutagenesis that some viral vectors present. These methods sometimes struggle with limited transduction rates as well as persistent gene expression problems.
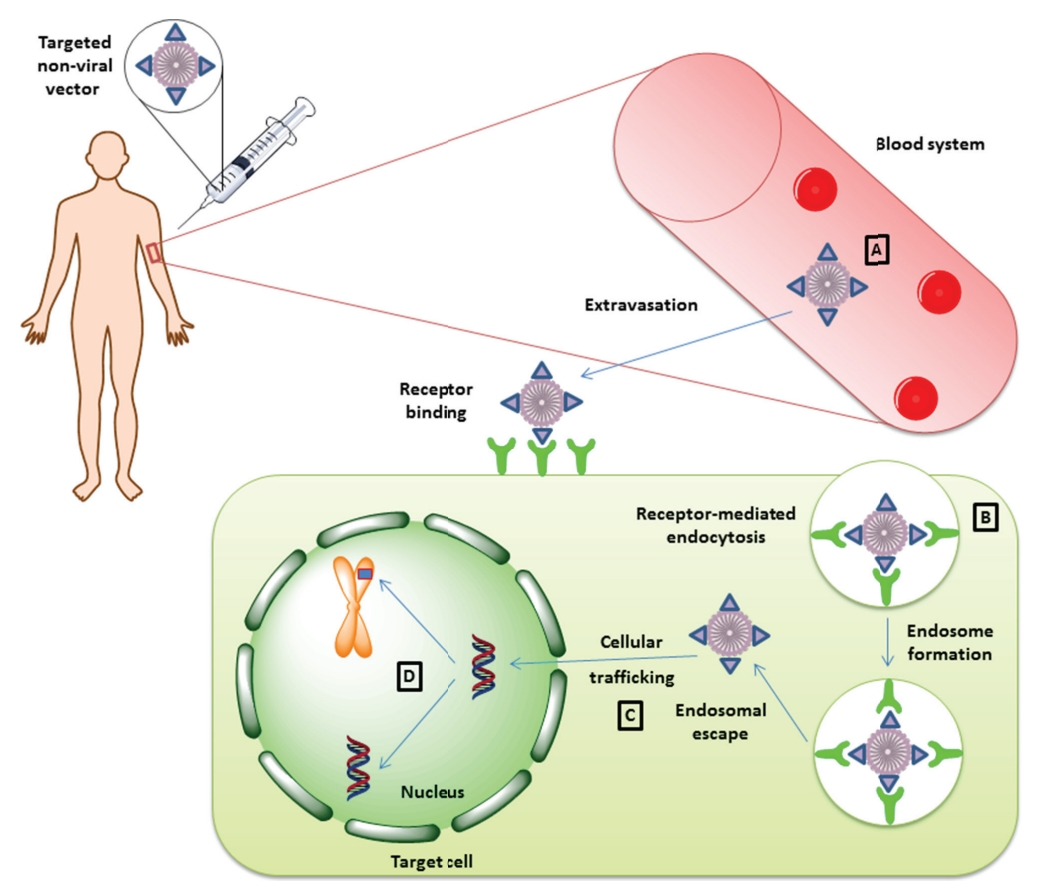 Fig. 2 Therapeutic gene delivery mediated by non-viral vectors2,7.
Fig. 2 Therapeutic gene delivery mediated by non-viral vectors2,7.
- Advantages and Disadvantages
Advantage of viral vectors-High transduction efficiency
Their main advantage lies in their superior transduction efficiency. The ability of viruses to infect cells and deliver their GM ensures that viral vectors deliver therapeutic genes efficiently into target cells. Lentiviruses possess the ability to infect both cells that divide and those that do not divide which allows them to function across a wide range of tissue types. Certain viral vectors, such as AAV, stand out for their capacity to sustain gene expression over extended periods which proves essential in the treatment of chronic diseases.
Disadvantages of viral vectors
The possible immune reactions present a significant problem with viral gene therapy. The human body's evolutionary defenses against viruses mean that viral vectors can produce immune reactions which potentially reduce therapeutic effectiveness and cause negative side effects.
The risk of insertional mutagenesis emerges when the viral vector integrates into the host genome disrupting normal gene function and possibly creating harmful mutations.
Viral vectors tend to have restricted payload capacity so they cannot transport large quantities of GM. The limited capacity of viral vectors poses a challenge when therapy requires the delivery of large genes or multiple genes at once.
Advantage of non-viral vectors
Non-viral vectors demonstrate a substantial benefit through their reduced potential to trigger immune responses when compared to viral vectors. Non-viral vectors escape immune system detection because they do not incorporate viral components.
Non-viral vectors have the capacity to transport bigger genetic payloads which proves useful for introducing large genes or multiple genes simultaneously. Non-viral vectors present a more cost-effective and simpler production process than their viral counterparts.
Disadvantages of viral vectors
Non-viral vectors demonstrate significantly reduced transduction efficiency in comparison to viral vectors. Non-viral vectors need higher doses to achieve effective gene delivery and expression since they struggle to reach high levels on their own.
The challenge of attaining effective cellular absorption together with successful endosomal escape remains a significant problem. GM delivered into cells needs to break out of the endosomes so it can move to the nucleus for gene expression. The therapeutic efficacy of non-viral vectors suffers due to their common failure to execute this process properly.
Criteria for Gene Therapy Vector Selection
- Safety of Vectors
Ensuring the safety of the vector remains the top priority in gene therapy applications. Comprehensive assessment of vectors is essential to confirm that they pose no substantial risk to patients. The potential for immune system reactions stands as one of the top issues. The human immune system detects and reacts strongly to viral vectors because they are considered foreign substances. When a vector causes an immune reaction, it results in inflammation and tissue damage while simultaneously diminishing the therapy's effectiveness. Adenoviral vectors trigger robust immune responses that restrict their repeated application and long-term therapeutic success.
Integrating viral vectors such as lentiviruses present a safety issue because they can cause insertional mutagenesis. The insertion of these vectors' GM into the host genome may disrupt nearby genes potentially causing harmful mutations which could trigger oncogenesis (cancer development). To determine vector safety profiles researchers must carry out thorough preclinical testing with animal models. Clinical trials should incorporate detailed monitoring protocols to identify any negative effects in human patients. The FDA and other regulatory bodies enforce strict guidelines which gene therapy vectors must meet to achieve high safety standards before receiving approval for use. The process of ensuring vector safety requires continuous monitoring and evaluation during both the development stages and application phases of gene therapies.
- Targeting of Vectors
Gene therapy requires vectors to precisely deliver therapeutic genes to diseased cells or tissues as an essential selection factor. Targeted delivery represents a fundamental approach to increase treatment effectiveness and reduce unintended biological effects. Therapeutic genes delivered by vectors to unintended cells or tissues result in off-target effects which cause unwanted side effects and diminish therapy effectiveness. Successful gene therapy for liver-related genetic disorders requires vectors that selectively target liver cells to avoid affecting other tissues. Targeting can be achieved through various strategies. Scientists modify the vector's surface by adding ligands or antibodies that attach specifically to receptors found on target cells. Engineered viral vectors can display specific peptides or antibodies which recognize cancer cell markers and lead the vector to infect cancer cells selectively. Therapeutic gene expression (TGE) can also be controlled by utilizing promoters that are active only in specific tissues. Promoters function as DNA regulatory components that determine the timing and location of gene expression. The therapeutic gene achieves expression solely in liver cells because the vector contains a liver-specific promoter that restricts gene expression to liver tissue even when the vector travels to different tissues.
- Expression Efficiency of Vectors
The success of gene therapy hinges on the level of TGE following its delivery. A gene therapy treatment achieves success when the vector delivers the therapeutic gene to target cells and ensures adequate gene expression levels for therapeutic effectiveness. The generation of therapeutic proteins at high levels demands efficient gene expression methods to effectively treat specific diseases. The measurement of transduction efficiency evaluates the ability of the vector to enter target cells and deliver GM into their nucleus. Viral vectors outperform non-viral vectors in transduction efficiency since they naturally possess cell-infecting abilities. While viral vectors belong to the same classification they exhibit differing levels of transduction efficiency. Lentiviruses demonstrate unique abilities to infect both dividing and non-dividing cells enabling them to function across multiple tissue types. Expression efficiency depends greatly on the strength of the promoter used in the vector system. DNA regions known as promoters start the process that converts genes into RNA before RNA gets translated into protein. Strong promoters trigger high expression levels whereas weak promoters result in poor protein production. Choosing appropriate promoters is fundamental to vector development for targeted therapeutic uses.
Optimization Strategies for Vectors
- Application of Gene Editing Technologies in Vector Optimization
The vector's safety stands as the top priority in gene therapy applications. Vectors require detailed evaluation to confirm they present no substantial risks to patients. A significant worry in gene therapy involves immune system responses to treatment vectors. The human immune system shows strong sensitivity toward external substances which encompasses viral vectors as well. An immune response triggered by a vector results in inflammation and tissue damage while weakening the therapy's effectiveness. Adenoviral vectors produce powerful immune responses which restrict their repeated application and sustained therapeutic benefits.
The risk of insertional mutagenesis represents a major safety issue especially in the case of integrating viral vectors such as lentiviruses. The integration of GM by these vectors into the host genome carries the risk of disrupting nearby gene functions which can produce harmful mutations or trigger oncogenesis (the development of cancer). To determine the safety profile of vectors, researchers need to conduct extensive preclinical testing which includes animal model studies. Clinical trials need careful design to detect any negative effects within human participants. The FDA sets strict rules which gene therapy vectors must follow to achieve necessary safety standards before receiving approval for clinical use. The continuous process of ensuring vector safety requires ongoing monitoring and evaluation during both development and application stages of gene therapies.
- Targeting of Vectors
Vector selection for gene therapy depends on the ability to precisely direct the vector to the affected cells or tissues. The specific delivery of vectors to targeted cells is necessary to enhance treatment effectiveness while reducing unintended effects. Vectors may deliver therapeutic genes to non-target cells or tissues leading to unintended side effects which diminish the therapy's overall success. When treating a genetic disorder of the liver, the gene therapy vector must target liver cells over other tissues for effective treatment. Targeting can be achieved through various strategies. To direct vectors to specific cells scientists attach ligands or antibodies to the vector surface which target receptors present on the desired cells. Scientists can modify viral vectors to carry peptides or antibodies which bind to cancer cells' unique surface markers so that these vectors target and infect cancer cells specifically. The TGE can be controlled through tissue-specific promoters which guide its activation. Promoters within DNA function as regulatory elements that determine both the timing and location of gene expression. The vector contains a liver-specific promoter which ensures TGE happens solely in liver cells regardless of other tissue exposure.
- Expression Efficiency of Vectors
Gene therapy has been transformed by gene editing tools like CRISPR/Cas9 which enable new methods for vector optimization. The CRISPR/Cas9 system functions as a powerful instrument for making exact changes to DNA sequences. Through applications in vector optimization gene editing methods enhance both the safety profiles and targeting abilities of vectors. CRISPR/Cas9 technology enables scientists to modify viral vectors by deleting sequences that could activate host immune responses or result in insertional mutagenesis. The vector achieves increased safety for gene therapy applications through the removal of its potentially harmful elements. Through gene editing scientists can add precise targeting elements directly into the genome of vectors. CRISPR/Cas9 technology enables the integration of sequences that produce ligands or peptides capable of binding target cell receptors. The modification boosts the vector's capacity for cell-specific infection which results in better targeting efficiency. Gene editing methods provide the capability to improve TGE levels. Adjusting the regulatory elements of the vector including promoters and enhancers enables precise control over gene expression levels and patterns to produce the intended therapeutic outcomes. CRISPR/Cas9 technology can swap a weak promoter for a stronger one to boost TGE levels.
- Application of nanotechnology in vector optimization
Nanotechnology represents an evolving field with groundbreaking approaches to enhance non-viral vectors used in gene therapy. Scientists use nanoparticles because their tiny size and unique characteristics enable them to enhance GM delivery and expression through engineering. Nanoparticles offer crucial protection mechanisms which prevent DNA from becoming degraded. The body's nucleases attack introduced GM because these enzymes break down DNA. DNA encapsulation in nanoparticles creates a protective barrier against degradative enzymes which maintains the GM's integrity until it reaches the target cells. Nanoparticles improve the efficiency at which cells take up GM. Scientists can alter nanoparticle surfaces to improve their interactions with cell membranes which helps the particles penetrate cells more easily. Nanoparticles can be coated with molecules that resemble natural ligands which cell surface receptors recognize leading to enhanced cellular uptake efficiency. Optimizing nanoparticles requires enhancing their ability to escape from endosomes. Nanoparticles become trapped within endosomes after entering cells and these small vesicles block GM from accessing the nucleus to be expressed. Nanoparticles equipped with membrane-disrupting elements enable GM to release into the cytoplasm which boosts chances of successful gene expression.
Case Studies
- Best Vector Selection for Cystic Fibrosis Gene Therapy
Cystic fibrosis (CF) results from CFTR gene mutations which cause the body to produce thick sticky mucus that builds up in the lungs and other organs. The delivery of a correct CFTR gene copy through gene therapy presents significant potential as a treatment for CF when administered to targeted cells. AAV vectors have become the preferred option for cystic fibrosis gene therapy in this treatment context. AAV vectors possess multiple attributes which perfectly qualify them for use in this treatment application. The main benefit of using these vectors stems from their minimal ability to provoke an immune response. AAV vectors generate weaker immune responses compared to other viral vectors which ensures their effectiveness during multiple treatments and long-term therapy. Comprehensive preclinical studies support the selection of AAV vectors for CF gene therapy. Research results show that AAV vectors can safely deliver the CFTR gene to airway epithelial cells in both animal models and human clinical trials. Researchers focus on optimizing AAV vectors for CF gene therapy through methods to boost transduction efficiency and cell-type targeting in airways while minimizing immune system reactions. The creation of AAV-based gene therapies for CF marks a major advancement in managing this serious genetic condition.
- Scientific Basis for Vector Selection
The choice of AAV vectors for cystic fibrosis gene therapy results from extensive scientific investigations that evaluate disease-specific needs together with target cell properties. CF manifests primarily as a lung condition and targets airway epithelial cells through a genetic mutation. Since these cells divide slowly or not at all, successful gene delivery requires the vector to transduce these cells without depending on cell division. AAV vectors become the preferred choice since their ability to infect cells ensures effective delivery of therapeutic genes to target cells. The minimal immunogenicity of AAV vectors stands as an essential element in CF gene therapy approaches. Environmental antigens continuously reach the lungs while the airway immune system maintains high activity levels. CF demands continuous genetic repair to produce significant therapeutic outcomes. The ability of AAV vectors to sustain gene expression over a long period matches the treatment objectives required to manage CF. Researchers can modify AAV vectors to direct them toward precise cell types found in the respiratory passages. The vector achieves selective therapeutic gene delivery to targeted cells by using elements that recognize unique airway epithelial cell markers while reducing unintended effects.
Conclusion
The development of successful gene therapies depends critically on both vector selection and optimization. The selection of a vector establishes the delivery effectiveness of therapeutic GM to target cells and ensures its safe integration and efficient expression for achieving therapeutic goals. Researchers frequently employ viral vectors in gene therapy since these vectors offer high transduction efficiency and sustainable gene expression. Advancements in gene therapy depend on the ongoing development and refinement of vector technologies. Researchers need to develop advanced vectors that merge the benefits of viral and non-viral systems while eliminating their individual weaknesses. Hybrid vectors that fuse elements from both viral and non-viral types demonstrate potential for enhanced performance. Personalized medicine approaches to gene therapy show significant potential for future advancements. Personalized treatment methods that adapt vector design and therapeutic approaches to match each patient's unique genetic and biological profile have the potential to deliver safer and more effective results. Artificial intelligence and machine learning tools speed up the process of finding the best vector designs and targeting strategies during vector optimization.
References
- Lundstrom, K. Viral Vectors in Gene Therapy: Where Do We Stand in 2023?. Viruses. 2023, 15, 698. https://doi.org/10.3390/v15030698.
- Chira, S.; Jackson, C. S.; Oprea, I.; Ozturk, F.; Pepper, M. S.; Diaconu, I.; Braicu, C.; Raduly, L.; Calin, G. A.; Berindan-Neagoe, I. Progresses towards safe and efficient gene therapy vectors. Oncotarget. 2015, 6: 30675-30703. https://doi.org/10.18632/oncotarget.5169.
- Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, M.H.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes. 2022, 13, 1370. https://doi.org/10.3390/genes13081370.
- Sainz-Ramos, M.; Gallego, I.; Villate-Beitia, I.; Zarate, J.; Maldonado, I.; Puras, G.; Pedraz, J.L. How Far Are Non-Viral Vectors to Come of Age and Reach Clinical Translation in Gene Therapy? Int. J. Mol. Sci. 2021, 22, 7545. https://doi.org/10.3390/ijms22147545.
- Bulcha, J.T.; Wang, Y.; Ma, H. Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther. 2021, 6, 53. https://doi.org/10.1038/s41392-021-00487-6.
- Toon, K.; Bentley, E.M.; Mattiuzzo, G. More Than Just Gene Therapy Vectors: Lentiviral Vector Pseudotypes for Serological Investigation. Viruses. 2021, 13, 217. https://doi.org/10.3390/v13020217.
- Distributed under Open Access license CC BY 4.0, without modification.
