Gene Therapy in the Treatment of Genetic Diseases
Current Treatment Status of Genetic Diseases
The contemporary genetic disease management paradigm features various treatment methods that are specifically tailored according to each disease's distinct features and severity. Managing the symptoms experienced by patients stands as the primary treatment approach for many genetic disorders. Medications used in treatment help manage symptoms including pain along with seizures and metabolic imbalances. Cystic fibrosis patients require daily chest physiotherapy alongside inhaled medications to manage respiratory issues while enzyme replacement therapies serve as treatments for metabolic disorders including Gaucher disease. Surgical interventions become necessary to fix structural abnormalities or manage complications on certain occasions. Even though these treatments are used they only offer temporary relief by addressing symptoms while failing to solve the underlying genetic issue. Progressive genetic disease treatments demonstrate their effectiveness limitations because no curative treatments exist and patients must continually battle worsening symptoms over their lifetimes. The demand for treatments that deliver more precise results and better effectiveness explains the rising interest in gene therapy.
What is a Gene Therapy
Through direct modification of genetic material in selected cells gene therapy introduces groundbreaking disease treatments. Gene therapy modifies cells through the introduction of new genetic sequences or changes to existing genetic information in order to fight against or prevent diseases. Gene therapy treats diseases by substituting faulty genes with functional genes at their genetic foundation. CRISPR-Cas9 technology together with viral vector delivery enables gene therapy to modify specific genes precisely. Scientists have successfully treated severe combined immunodeficiency (SCID) by using gene therapy to insert a functional gene into bone marrow cells which resolves the genetic disorder and restores normal immune function. Gene therapy delivery methods available to medical professionals include tissue injections and intravenous administration along with cell modification outside the body prior to reintroduction. The field of gene therapy has made great strides due to improvements in molecular biology and genetic engineering together with delivery technologies resulting in a fast-developing promising medical field.
Potential of Gene Therapy
Gene therapy presents enormous transformative possibilities. Gene therapy creates opportunities to treat genetic conditions which have been historically untreatable. Gene therapy offers potential long-term or permanent treatments by addressing the genetic basis of disorders which decreases the necessity for ongoing management of symptoms. Recent advancements in gene therapy have demonstrated the ability to restore sight in patients facing inherited retinal diseases who would otherwise face blindness. Gene therapy is being explored for complex diseases such as cancer which evolve through harmful genetic mutations alongside its traditional use for monogenic disorders. Gene therapy for cancer treatment works by strengthening the immune system to recognize cancer cells for destruction or seeks to target cancer cells directly. Gene therapy shows potential treatment responses for neurological conditions such as Huntington's disease and neurodegenerative diseases like Alzheimer's because genetic factors play a critical role in these disorders. The progress in gene therapy research demonstrates its transformative potential in medical science to improve the lives of millions who suffer from genetic diseases.
Mechanism of Gene Therapy in Genetic Diseases
- Gene Replacement Therapy
Gene replacement therapy serves as a basic strategy within gene therapy by inserting a normal gene variant to substitute a faulty gene to treat genetic disorders. Gene replacement therapy demonstrates significant success rates when treating recessive monogenic diseases that stem from one defective gene. Adeno-associated virus (AAV) serves as the primary vector for gene replacement because its small size along with its non-pathogenic nature and limited immune response make it ideal. Gene replacement therapy operates through multiple essential processes. The therapeutic gene is added to a viral vector before this modified vector delivers it to the patient's cells. The viral vector transports the therapeutic gene to the cell nucleus after entering the cell so it can be expressed there. The newly introduced gene replaces the defective gene's lost function which helps return cells to normal operation while lessening disease symptoms. Gene replacement therapy faces multiple significant obstacles. The delivery of genes through viral vectors faces a major limitation because of the maximum size threshold for the genes that can be transported. The current viral vectors cannot accommodate large genes which limits the number of treatable diseases.
- Gene Silencing Therapy
Gene silencing therapy aims to disable damaging genes and functions as a treatment option for diseases triggered by gain-of-function mutations. The technique combines multiple methods to suppress gene expression through RNA interference (RNAi) and epigenetic changes. RNAi acts as an effective gene silencing technique through which small RNA molecules like short interfering RNA (siRNA) and microRNA (miRNA) target and degrade specific messenger RNA (mRNA) molecules. The Dicer enzyme processes double-stranded RNA within cells into siRNA fragments through cleavage. The RNA-induced silencing complex (RISC) incorporates small RNA fragments which identify complementary mRNA sequences for degradation to stop gene expression. The gene silencing process unfolds through an epigenetic pathway that includes both DNA methylation and histone modification. Changes to chromatin structure block transcription machinery access to genes which inhibits gene expression. Gene expression silencing therapies demonstrate potential effectiveness in treating both Huntington's disease and multiple forms of cancer. Cancer therapy can benefit from oncogene silencing which stops cancer cell growth and triggers apoptosis. The delivery process and precision targeting of gene silencing components still pose significant challenges. The effectiveness and safety of these therapies are limited by off-target effects and immune responses to therapeutic agents.
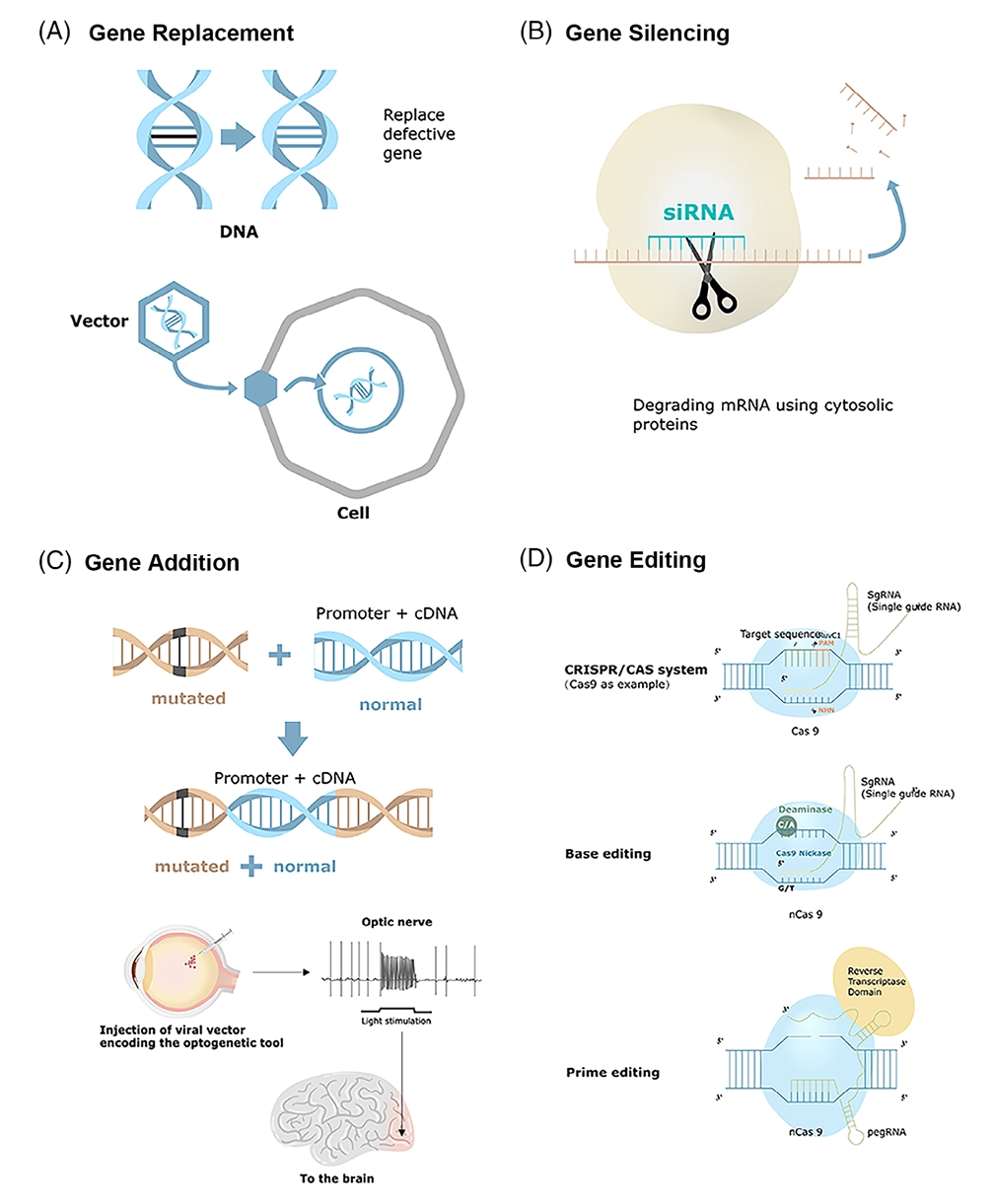 Fig. 1 Different gene therapy strategies1,6.
Fig. 1 Different gene therapy strategies1,6.
Types of Vector in Gene Therapy
Vectors function as delivery tools in gene therapy to transport therapeutic genes into target cells. Vectors can be broadly classified into two categories: viral vectors and non-viral vectors. The selection of vectors depends on the unique demands of the therapy because each vector type presents distinct benefits and drawbacks.
- Viral Vectors
Retroviral vectors
Retroviruses are complex enveloped RNA viruses that contain a diploid ssRNA genome and possess at least four essential genes named gag, pro, pol, and env. The gag gene produces the primary structural polyprotein needed for forming noninfectious and immature viral-like particles. The pro gene produces the viral protease which works to promote the maturation of viral particles. The pol gene creates reverse transcriptase, RNase H, and integrase proteins while env encodes the surface glycoprotein and transmembrane proteins which assist in receptor binding and membrane fusion. The defining property of RV and retroviral vectors is their capability to insert themselves into host DNA. Researchers use retroviral vectors extensively to deliver therapeutic genes in gene therapy applications and clinical treatments for monogenic disorders and cancer as well as infectious diseases because these vectors ensure stable and efficient transgene expression in patients. Retroviruses have numerous advantages over other vectors. Retroviral vectors excel because they can convert their ssRNA genome into a dsDNA molecule that integrates into the target cell genome in a stable manner. Retroviral vectors have the ability to make lasting modifications to the host cell nuclear genome.
Lentivirus vectors
Lentiviruses which are a form of RV contain a single-stranded positive-sense RNA sequence that transforms into DNA and integrates into the host genome leading to persistent infection. The majority of lentiviral vectors (LVVs) originate from HIV-1 while preserving their ability to integrate into host cell genomes. LVVs are built upon the full wild-type HIV genome which includes its entire set of genes and regulatory elements. The widespread use of lentiviral vectors in clinical applications stems from their enhanced ability to transduce nonproliferating or slowly dividing cells including CD34+ stem cells. The application of lentiviral vectors for gene delivery into CD34+ HSCs has resulted in successful treatments for genetic disorders such as β-thalassemia, X-linked adrenoleukodystrophy, metachromatic leukodystrophy and Wiskott-Aldrich Syndrome.
Adenovirus vectors
Adenoviruses are DNA viruses with double-stranded genomes that measure between 34 kb and 43 kb and they utilize alternative splicing for the generation of both sense and antisense genes. Adenovirus initiates its genomic transcription in the primary regions E1A, E1B, E2, E3, and E4 to generate proteins that activate further viral transcription and adapt the host cell environment for effective viral propagation. The late regions L1 through L5 originate from a transcript that undergoes alternative splicing. Adenoviral vectors demonstrate substantial benefits in comparison to other viral gene delivery systems. The primary reason adenoviral vectors stand out in gene delivery in vivo is because human cells express both the primary AV receptor and the secondary integrin receptors which facilitate viral entry. AV vectors can easily infect human cells which results in high transgene expression levels. Gutless adenoviral vector development enables us to bypass the antiadenoviral vector immune response. AV vectors stand alongside AAV and lenti/retrovirus systems as one of the three principal viral vector types utilized in gene therapy.
Adeno-associated virus (AAV) vectors
Nonpathogenic parvoviruses known as AAV vectors have a linear ssDNA genome that spans approximately 4.7 kb and contains two inverted terminal repeats of 145 nucleotides positioned at both genome ends. The virus does not have polymerase genes of its own and must use polymerases from host cells to replicate its genome. The AAV genome structure features three open-reading frames (ORF) which are positioned between two ITRs. The ITRs serve dual roles as the viral origin of replication point and packaging signal sequence. The rep ORF encodes 4 nonstructural proteins. The structural proteins function as essential components for viral replication and transcriptional control while also enabling genomic integration and virion assembly. The viral capsid consists of a 60-mer repeat unit formed by three structural proteins VP1, VP2, and VP3 encoded by the cap ORF. The assembly-activating protein emerges from an alternate reading frame located in the cap gene which directs AAV capsid proteins to the nucleolus while participating in the capsid assembly process. AAV stands as one of the primary vectors employed in gene therapy applications. AVV remains a preferred vector in gene therapy efforts because it delivers stable and effective transgene expression across multiple cell types within essential tissues including the liver, muscle, retina, and central nervous system.
Herpesviral vectors
Herpes simplex virus consists of an enveloped structure with dsDNA genome that exceeds 150 kb and includes long and short unique segments named UL and US as well as inverted repeated sequences TRL/IRL and TRS/IRS. The HSV genome contains about 90 genes and almost half of these genes are not essential which allows them to be removed from recombinant vectors. The neurotropic HSV-1 virus shows multiple essential adaptations for functioning within the nervous system. Rational exploitation of neurological adaptations is possible during the development of gene therapy vectors for neurological purposes. Genetic engineers have developed HSV vectors which use latency-active promoters LAP1 and LAP2 to achieve sustained expression lasting up to 300 days in the peripheral nervous system. HSV vectors now serve as tools for CNS applications. The development of nontoxic replication-defective HSV vectors requiring IE gene deletions (ICP4, ICP22, ICP27) remains crucial due to wild-type HSV's potential to cause encephalitis. The latest advancements in HSV-1 technology enable scientists to use HSV-1 as vaccine vectors to deliver foreign antigens to the immune system.
- Non-Viral Vectors
Lipid nanoparticles (LNPs)
The structure of LNPs consists of lipids which function to hold genetic material and deliver it to specific cells. These particles release their contents after merging with cellular membranes. These materials demonstrate biocompatibility properties while reducing toxic effects of drugs and enabling the escape from endosomes. It is simple to modify these particles to enhance both their targeting ability and delivery efficiency. The loading capacity of LNPs remains low which necessitates optimization for improved stability and efficacy.
Polyplexes
The formation of polyplexes occurs when cationic polymers establish electrostatic connections with genetic material to create nanoscale structures. Polyplexes exhibit minimal toxicity while avoiding immune responses and demonstrate broad functional adaptability. They defend small RNAs against degradation while enabling their release inside cells. Polyplex delivery effectiveness of genetic material depends on both the particular polymer and the target tissue being used.
Naked DNA (plasmid)
Scientists have extensively studied naked DNA because it is pure DNA without protein attachments as a method to transfer genes to numerous tissues including skin cells and liver cells. The technique requires injecting DNA straight into target tissues to transfer genes that range from 2 to 19 kilobases in size. Naked DNA-based gene transfer is both safe and uncomplicated but can only be used in specific applications like DNA vaccination. Researchers have documented sustained gene expression in skeletal muscle following injections that persisted beyond a 19-month period. Naked DNA shows limited effectiveness in gene delivery because fewer than 1% of total myofibers develop transgenic expression after one injection. Multiple injections of naked DNA enhance transfection success rates which enables the use of this method for specific gene therapy applications.
Preclinical Research in Gene Therapy
- Research methods and models
In vitro models
Cell lines or primary cells from relevant disease tissue sources are the typical subjects of in vitro studies. Cancer research utilizes tumor cell lines to assess how well gene therapy vectors perform. These cell cultures enable researchers to assess the delivery efficiency of genes and determine both expression levels and functional correction of therapeutic genes. High-throughput screening tests multiple gene therapy constructs or delivery systems in parallel. Performance evaluation of vectors and gene-editing tools in large-scale assays enables researchers to identify their most effective candidates. Scientists perform in vitro validation of CRISPR-Cas9 methods to achieve precise genetic modifications while reducing unintended mutations. The analysis of edited cells includes checking the presence of target genetic modifications and identifying any unintended mutations.
In vivo models
Animal models serve as critical tools to examine how gene therapy affects living organisms biologically. The most frequently utilized animal species in research experiments is mice because they share strong genetic parallels with humans while remaining both economical and having a fast reproductive cycle. Studies of complex diseases make use of non-human primates because they share close genetic ties with humans. The research employs diverse animal model types which consist of inbred strains together with disease induction models and xenograft models and genetically engineered models. Xenograft models study disease progression and treatment responses by transplanting human cells or tissues into animals with compromised immune systems. Researchers employ molecular biomarkers to evaluate the effectiveness of gene therapy treatments. MRI, PET, and bioluminescence imaging techniques enable researchers to monitor therapeutic gene delivery and expression within animal models.
- Determine the Viability of Gene Therapy Approaches
Efficacy analysis
The level of gene expression after therapy treatment is measured in preclinical studies through methods such as qPCR and Western blotting. Target tissues show therapeutic gene expression levels that allow disease correction in gene replacement therapy for genetic disorders. Functional tests measure if the treatment gene successfully addresses disease symptoms. The return to standard metabolic function in metabolic disorders can be verified through assessments of distinct metabolite concentrations and enzyme activities. Animal studies use behavioral and physiological evaluations to measure the comprehensive effect of gene therapy treatments. Motor function and cognitive tests measure symptom improvement in neurological diseases.
Safety analysis
Preclinical studies track immune responses to gene therapy vectors throughout experimental processes. The process includes measuring both antibody production and immune cell activation levels as part of the analysis. Research with viral vectors examines how immune responses might restrict treatment effectiveness or produce harmful effects. Next-generation sequencing (NGS) serves as a diagnostic tool to detect genetic modifications that occur unexpectedly during gene-editing treatments. Optimizing gene-editing tools through this methodology reduces associated risks. The safety profile of gene therapy is determined through comprehensive toxicity studies that involve histopathological analysis and evaluations of liver and kidney function. Researchers maintain detailed records of all toxicity indicators and adverse reactions for thorough examination.
Challenges in Gene Therapy
- Gene Delivery and Activation
Gene therapy faces a major obstacle which involves delivering therapeutic genes into target cells and activating them effectively. Successful treatment of many genetic disorders depends on delivering the therapeutic gene to numerous cells within specific tissues so they can express it effectively. This process involves several critical steps: Therapeutic success depends on delivering the gene to the appropriate cells and then activating it so that it produces the necessary protein while maintaining this expression over time. The effectiveness of gene therapy is reduced when cells turn off genes that show abnormal activity patterns. The possibility of delivering therapeutic genes to unintended targets represents another major problem. Patients face unintended health consequences when therapeutic genes are delivered to incorrect cells or tissues. Incorrect targeting during gene therapy may lead to the integration of the gene into reproductive cells which could then be inherited by future generations. The long-term effects of these genetic modifications remain unknown which generates significant ethical and safety concerns.
- Immune Response and Immunotoxicity
The human immune system functions to identify and eliminate foreign substances which creates significant obstacles for effective gene therapy application. Therapeutic gene or vector introduction into the body can activate immune responses that decrease treatment effectiveness and produce serious side effects. The use of adenoviral vectors in gene therapy treatments typically results in significant immune responses that cause inflammation along with the possibility of organ damage. The immune reactions from gene therapy trials can sometimes lead to fatal outcomes demonstrated by Jesse Gelsinger's death from an excessive immune response during his trial.
- Insertional Mutagenesis
Integrating viral vectors like retroviruses and lentiviruses pose a significant insertional mutagenesis risk in ex vivo gene therapy applications. These vectors introduce their genetic material into the host DNA which may disrupt regular gene operations or trigger oncogenes that could cause cancer development. Some patients treated in early gene therapy trials for severe combined immunodeficiency (SCID) developed leukemia because of insertional mutagenesis. Researchers have created new vectors to mitigate risk but continuous long-term patient monitoring remains essential for safety assurance.
- Vector Limitations
Selecting the right vector for gene therapy is essential yet every vector type presents distinct limitations. Viral vectors deliver genes effectively yet their ability to trigger immune responses and limited packaging capacity prevent larger therapeutic genes from being delivered. Lipid nanoparticles and polyplexes as non-viral vectors show reduced immunogenicity but suffer from poor transfection efficiency requiring larger doses that may lead to increased toxicity levels. The production scale-up and manufacturing of vectors including lentiviral vectors present significant technical challenges and high expenses.
Future Directions in Gene Therapy
- Application of new technologies
CRISPR-Cas9 and gene editing
Researchers consider CRISPR-Cas9 a highly effective gene-editing tool because it enables precise genetic alterations within the genome. Genetic editing technology permits direct DNA mutation correction which could eliminate single-gene defect diseases. Recent studies have demonstrated that CRISPR technology can successfully correct genetic mutations in conditions like sickle cell anemia and Duchenne muscular dystrophy. A significant concern regarding CRISPR technology is its possibility of causing unintended genomic edits. The development of high-fidelity Cas9 variants and base editing technology advancements are effectively decreasing these risks. Base editors enable direct base-to-base DNA conversion without producing double-strand breaks which increases both safety and precision.
Advanced vector development
Current research efforts aim to create viral vectors that demonstrate enhanced safety features alongside diminished immunogenicity. Research efforts are creating modern AAV vectors that focus on specific tissues while also reducing the potential for insertional mutagenesis. Lipid nanoparticles and polymeric vectors belong to non-viral delivery systems which are becoming popular because they produce fewer immune responses and can be manufactured at large scales. Scientists are optimizing vectors to improve their effectiveness in delivering genetic material and their ability to enter cells. Researchers are utilizing synthetic biology techniques to develop specialized vectors along with delivery systems. Customizable therapeutic options for different diseases and patient demographics allow for individualized gene therapy treatments.
mRNA therapeutics
The main advantage of mRNA-based therapies lies in their ability to temporarily produce therapeutic proteins. The therapeutic approach becomes essential when permanent gene expression is inappropriate or dangerous. mRNA vaccines proved effective against COVID-19 which led scientists to investigate their use in treating both infectious diseases and cancer. The primary technical obstacle in mRNA therapies lies in their effective delivery and preservation. Advanced lipid nanoparticles and alternative delivery systems are currently under development by researchers to safeguard mRNA molecules and promote their cellular uptake.
- Importance of interdisciplinary collaboration
The identification of disease-related genetic mutations and creation of precise treatments depends heavily on the expertise of geneticists and molecular biologists. The team maps the human genome and seeks to discover how genes function and identify therapeutic targets. The development of gene therapy delivery systems depends on the expertise of bioengineers and nanotechnologists. Their research focuses on producing both viral and non-viral delivery vectors and sophisticated materials including nanoparticles and liposomes to guarantee the effective and secure transfer of therapeutic genes. The work of clinicians and pharmacologists proves essential when converting laboratory findings into practical clinical uses. Clinical trials are designed and conducted to evaluate both the safety and effectiveness of gene therapies in patient treatments. The ethical and legal issues involved in gene therapy require the expertise of ethicists and regulatory specialists. These experts develop guidelines and policies to ensure ethical and responsible use of gene therapies specifically for germline editing and human enhancement applications.
References
- Liu, F.; Li, R.; Zhu, Z.; Yang, Y.; Lu, F. Current developments of gene therapy in human diseases. MedComm. 2024, 5:e645. https://doi.org/10.1002/mco2.645.
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Tesliuk, M.G.; Telegeev, G.D. Current State of Human Gene Therapy: Approved Products and Vectors. Pharmaceuticals. 2023, 16, 1416. https://doi.org/10.3390/ph16101416.
- Lundstrom, K. Viral Vectors in Gene Therapy. Diseases. 2018, 6, 42. https://doi.org/10.3390/diseases6020042.
- Soufizadeh, P.; Mansouri, V.; Ahmadbeigi, N. A review of animal models utilized in preclinical studies of approved gene therapy products: trends and insights. Lab Anim Res. 2024, 40, 17. https://doi.org/10.1186/s42826-024-00195-6.
- Ay, C.; Reinisch, A. Gene therapy: principles, challenges and use in clinical practice. Wien Klin Wochenschr . 2024, 1-11. https://doi.org/10.1007/s00508-024-02368-8.
- Distributed under Open Access license CC BY 4.0, without modification.
