siRNA in the Immune System-Modulating Immune Responses
Introduction of siRNA in Immune System
The RNA interference mechanism directed by small interfering RNAs represents an effective biological tool because it can silence specific genes through targeted expression suppression. The use of siRNA-directed RNAi represents an effective mechanism to perform gene silencing within mammalian cells. The extreme specificity of siRNA to knock down target genes through analogous mRNA degradation serves as one of the key reasons why siRNA stands out as an amazing biological tool. The application of RNAi faces limitations due to non-specific effects which encompass innate immune activation and suppression of unintended target genes. Mammalian innate immune systems activation serves as the most common non-specific effect from both cytoplasmic and endosomal pathways. The immune cells that activate the Toll-like receptor (TLR) pathway control the activation process. The relationship between siRNA sequences and these pathways relies on both the specific Toll-like receptor that is activated and where it is located. siRNAs penetrate the cytoplasm and activate non-immune cells through interactions with RNA sensors including retinoic acid-inducible gene I.
Immune System Activation by siRNA
Nanostructures utilized in delivery systems must possess the ability to evade detection by the immune system. The achievement of therapeutic success with siRNA design depends on maintaining balanced immune activation. The therapy requires triggering an effective immune response for optimal therapeutic effects while avoiding excessive immune activation that leads to side effects. Scientific research demonstrates that siRNA therapies delivered systemically can activate the innate immune system leading to increased levels of inflammatory cytokines including TNF-alpha, IL-6, and interferons primarily IFN-alpha. Peripheral mononuclear cells from human blood exhibit high IFN-alpha production by plasmacytoid dendritic cells when induced by siRNA through in vitro assays. The TLR pathway regulation within the innate immune system requires further investigation during siRNA therapy application. The TLR pathway shows the most prevalent non-specific effects which can restrict siRNA therapy applications. The consequences of TLR pathway alterations should be examined through non-clinical studies and clinical trials since these changes may affect siRNA therapy.
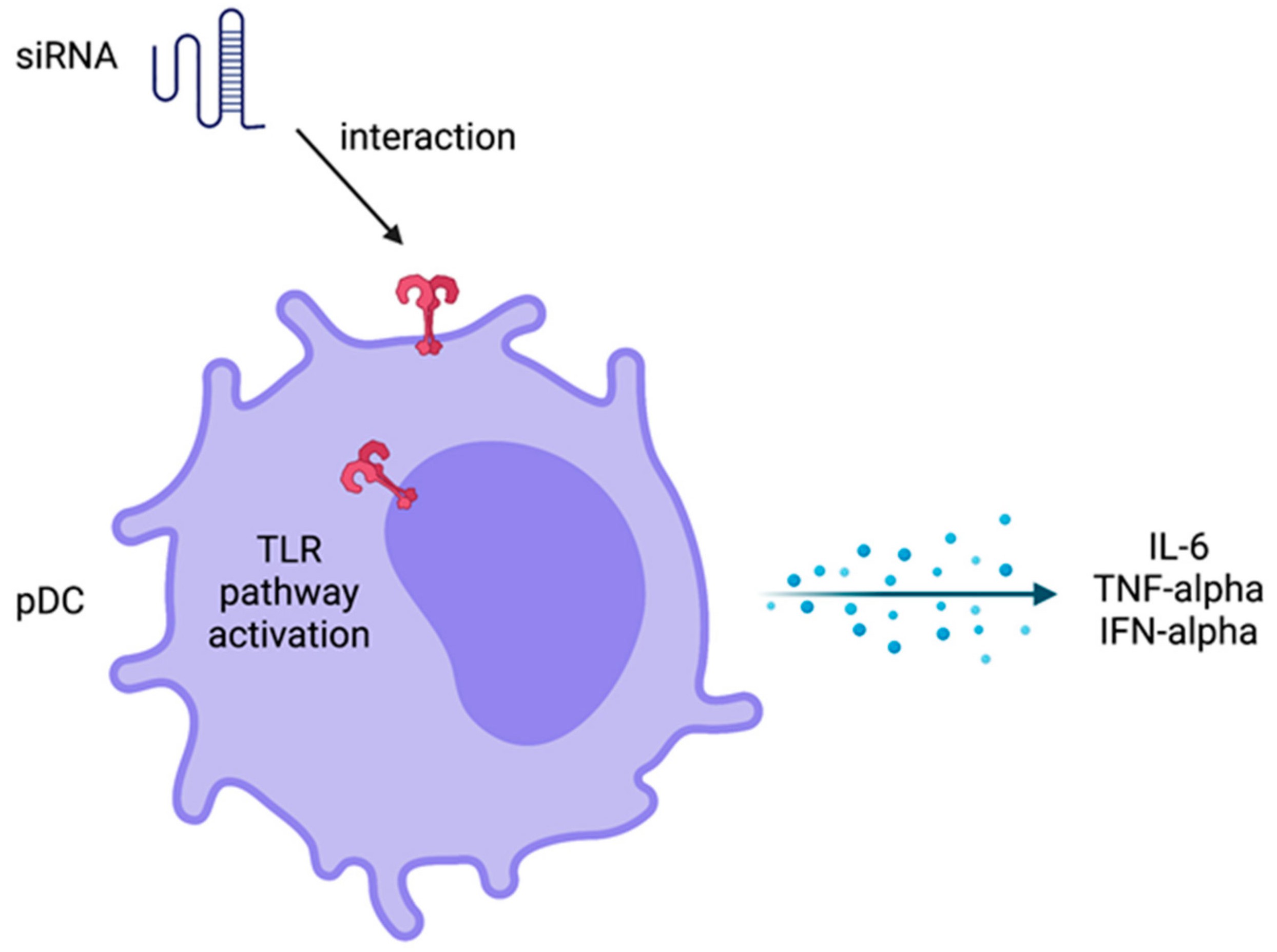 Fig. 1 Innate immune response activation by siRNA1,6.
Fig. 1 Innate immune response activation by siRNA1,6.
Mechanism of RNA interference in immune cells
Cells receive siRNA molecules by transfection methods or delivery systems such as lipid nanoparticles and exosomes. The RNA-induced silencing complex (RISC) processes siRNA molecules after they enter the cell. The siRNA molecules direct RISC toward matching mRNA sequences which undergo degradation and stop protein production. Researchers achieve accurate regulation of immune signaling pathway gene expression through this specific targeting degradation method. siRNA achieves control over the immune response by turning off specific genes. siRNA directed against inflammatory cytokines including TNF-α and IL-6 demonstrates therapeutic potential by decreasing inflammatory responses in rheumatoid arthritis patients. siRNA technology enables researchers to suppress genes that trigger immune cell activation or apoptotic processes which could lead to new treatments for autoimmune diseases and cancer.
siRNA fucntion in Immune Cell Regulation
- Targeting Cytokines and Chemokines
Research demonstrates that siRNA effectively targets pro-inflammatory cytokines like IL-6 and IL-1beta which serve important functions in initiating inflammation across different diseases. siRNA functions by degrading cytokine mRNAs which leads to a reduction in cytokine production and a consequent decrease in inflammatory responses. The application of siRNA against IL-6 results in substantial IL-6 level reduction in LPS-activated macrophages which leads to diminished inflammatory responses. siRNA designed to target IL-1beta results in reduced inflammation within rheumatoid arthritis models according to research findings.
The therapeutic success of siRNA depends on its efficient delivery to immune cells. Researchers developed multiple delivery systems to improve siRNA stability and uptake within immune cells. Lipid nanoparticles (LNPs) serve as a delivery system that can encapsulate siRNA to prevent its degradation. Through the inclusion of targeting ligands scientists can design LNPs to direct their delivery to specific immune cells such as macrophages or dendritic cells. Amphiphilic dendrimers represent an innovative delivery method which allows siRNA to reach primary immune cells like T cells, microglia and NK cells efficiently with minimal toxicity. These delivery systems transport siRNA to target cells and successfully silence the targeted genes.
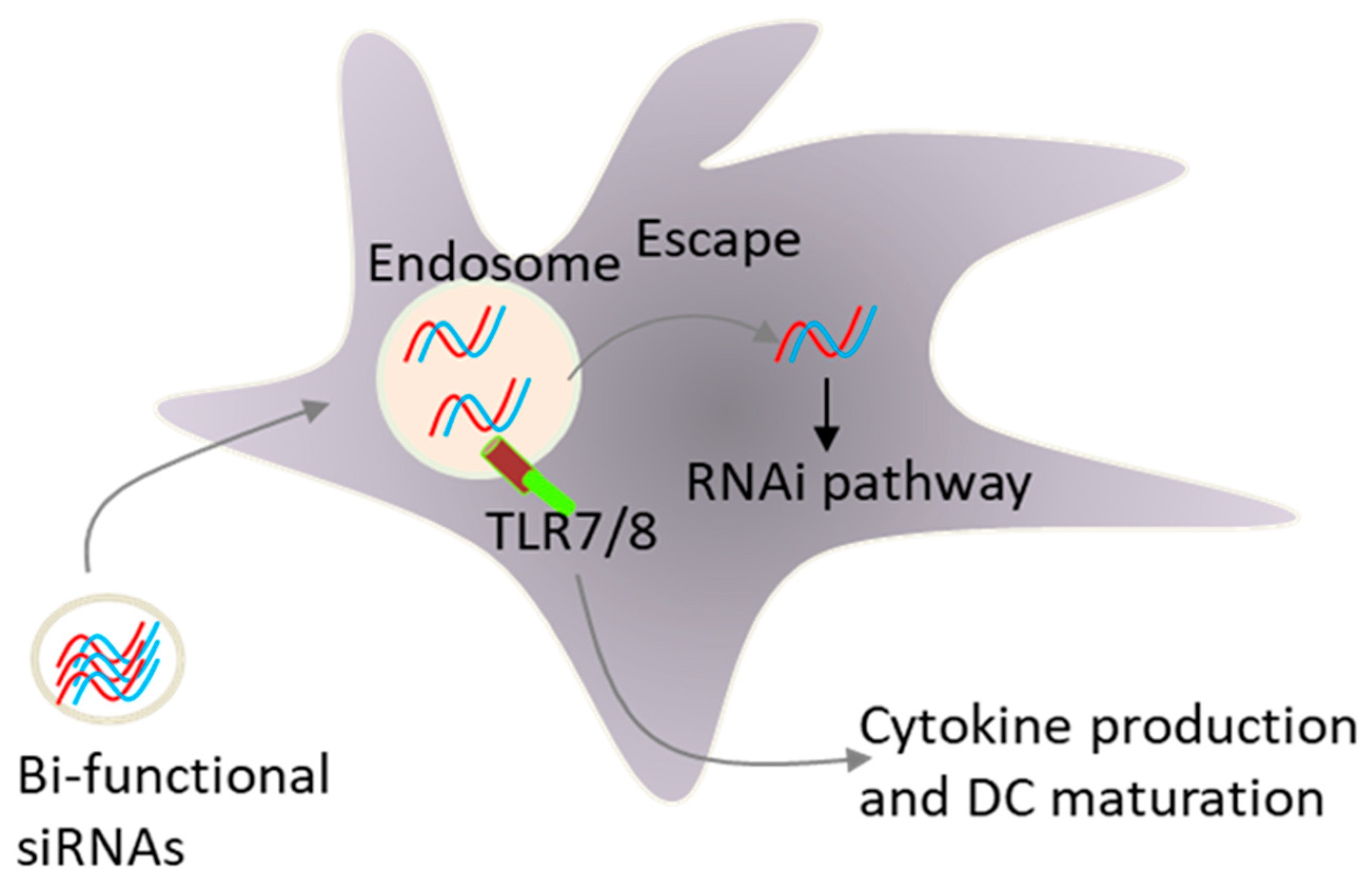 Fig.2 Combined gene silencing and Toll-like receptor TLR activation by a single siRNA sequence2,6.
Fig.2 Combined gene silencing and Toll-like receptor TLR activation by a single siRNA sequence2,6.
- Regulation of Immune Cell Activation
The use of siRNA to silence genes that regulate T cell and B cell activity presents a promising approach for treating autoimmune diseases and cancer. Research demonstrates that siRNA which targets the CTLA-4 gene promotes the activation and multiplication of T cells by decreasing inhibitory receptor expression. The efficacy of cancer immunotherapy improves when siRNA targeting the PD-1 gene prevents T cell exhaustion. The use of siRNA against the Bcl-2 gene in B cells leads to increased apoptosis which results in a decreased production of autoantibodies in autoimmune diseases.
siRNA application for immune cell activation modulation leads to notable changes in immune cell proliferation and function. When CTLA-4 expression is reduced in T cells there is an increase in T cell proliferation together with enhanced effector functions which include cytokine production and cytotoxic activity. T cells demonstrate improved survival and functionality within cancerous environments when PD-1 expression declines which leads to increased cancer cell destruction. The silencing of Bcl-2 survival genes through siRNA in B cells diminishes autoreactive B cell populations which leads to a reduced production of pathogenic autoantibodies. Targeted methods allow for exact regulation of immune responses which may enhance results for multiple immune-related disorders.
siRNA in Autoimmune Diseases
Animal Model Research on Lupus and Rheumatoid Arthritis
- Systemic Lupus Erythematosus (SLE)
A recent study created an advanced delivery platform with PEGylated TAT peptide-cationic liposomes to transport anti-HMGB1 siRNA together with dihydroartemisinin (DHA) to mice prone to lupus. The delivery system achieved substantial suppression of HMGB1 expression which resulted in reduced autoantibody production and decreased disease symptoms. The treatment achieved a 70% decrease in anti-dsDNA antibodies along with notable renal pathology improvements demonstrated by lower glomerular deposits and proteinuria. A separate study applied a lipid nanoparticle (LNP) system to deliver siRNA that targets the PTPN22 gene which plays a role in autoimmunity-related pathophysiological processes. The treatment approach successfully prevented the progression of lupus in the animal model because treated mice exhibited disease severity scores that were 60% lower than those of untreated controls.
- Rheumatoid Arthritis (RA)
siRNA that targets NF-κB p65 demonstrates potential therapeutic effects for RA treatment. Researchers developed an LMW PEI–cholesterol–PEG (LPCE) delivery micelle system to transport NF-κB p65 siRNA. The system achieved control over excessive inflammatory cells in joint cavities to slow disease progression. The treatment produced a 50% decrease in joint swelling and a substantial reduction in pro-inflammatory cytokines such as TNF-α and IL-6. Scientists utilized a cell-permeable peptide-conjugated liposome-polycation-DNA (LPD) complex known as CCP-LPDR for delivering RRM2 siRNA into rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS). The therapy suppressed RA-FLS proliferation while decreasing TNF-α and IL-6 cytokine levels. Treatment led to a 70% decrease in joint inflammation and produced substantial histological score improvements in the mice.
- siRNA in Inflammatory Bowel Disease
Researchers engineered MPEG-PCL-CH2R4H2C nanomicelles as delivery vehicles for siRNA that targets NF-κB in mice with ulcerative colitis. The treatment produced lower intestinal permeability along with diminished inflammation within the experimental model. The treatment decreased disease activity index (DAI) scores by 60% while substantially improving histological scores which demonstrated less inflammation and tissue damage. Researchers applied silica-coated calcium phosphate nanoparticles (CaP/PEI-Dy734/siRNA/SiO2) as a delivery system for NF-κB p65-specific siRNA in another study. The system reduced NF-κB-related protein expression and inflammatory cytokine levels in the colon of UC model mice. The colonic inflammation in treated mice decreased by 70% which was demonstrated by lower concentrations of TNF-α and IL-6.
siRNA in Infection Control
- Targeting Viral Genes in HIV and Influenza
The advancement of siRNA technology provides a precise and flexible method to target viral RNA and stop viral replication. Researchers design siRNA by choosing viral genome sequences vital to replication and function. Scientists created siRNAs to target HIV's tat, rev, and gag genes because these genes play a fundamental role in viral transcription and protein production. Influenza research has seen the development of siRNAs aimed at the viral polymerase complex components PA, PB1, PB2 and the nucleoprotein NP because these elements are critical for viral RNA replication and assembly. The siRNAs are created to match the viral mRNA sequence thereby achieving high precision and minimizing unintended off-target activity.
- siRNA in Bacterial Infections
siRNAs fail to silence bacterial genes because bacteria do not rely on host cell replication mechanisms. The bacterial pathogens Mycobacterium tuberculosis (MTB), Listeria monocytogenes, Mycobacterium fortuitum, S. typhimurium and Yersiniaceae depend on host cell structures to successfully invade their targets. The cellular entry of bacteria into host cells can be stopped by using siRNA to turn off invasion-related genes SEC22A, Rab1B, and VPS33B. Researchers developed specific siRNA molecules to target both urease B subunit (ureB) and cytotoxin-associated gene A (CagA) genes in Helicobacter pylori during a recent in vitro investigation. The virulence factors are crucial contributors to the development of gastric cancer linked to H. pylori infection. The research demonstrated that using siRNA to target ureB and cagA genes represents a novel approach for stopping urease activity while simultaneously decreasing inflammation and colonization levels. The use of siRNA speeds up the eradication of microorganisms through inflammation control. siRNA achieves TNF-α reduction in inflammatory conditions such as sepsis by targeting excessive levels of the molecule. Glutamine synthetase and β-hexosaminidase enzymes which participate in Mycobacterium cell wall biosynthesis and peptidoglycan hydrolase function represent potential siRNA silencing targets.
Future Directions of siRNA in Immune Modulation
- Modification to enhance performance
siRNA shows great potential for immune modulation as ongoing improvements in stability and delivery systems alongside target selection broaden its application scope. SiRNA stability is increased with chemical modifications like 2′-O-methyl and 2′-fluoro linkages as well as phosphorothioate linkages which decrease immune responses together with delivery systems such as lipid nanoparticles and GalNAc-conjugated siRNAs that boost cellular uptake and targeting accuracy. Recent improvements allow siRNA to better control immune system reactions. Through its ability to directly target long non-coding RNAs (lncRNAs), siRNA technology creates novel treatment methods for diseases driven by these RNA molecules. siRNA's capability to target conserved viral sequences makes it a versatile tool in combating emerging viral threats including SARS-CoV-2 and its variants. The targeted siRNA method applies to autoimmune diseases by selectively suppressing pro-inflammatory cytokines such as TNF-α and IL-6 to provide a more accurate option than general immunosuppressive treatments.
- Combination therapy
siRNA enhances cancer immunotherapy by targeting immune checkpoint proteins PD-1 and CTLA-4 for silencing which strengthens the immune system's capacity to destroy cancer cells. The therapeutic impact of siRNA broadens when it is paired with small molecules or combined with antibodies and vaccines in combination therapies. The combination of siRNA technology with personalized medicine through genomics and bioinformatics development produces treatments targeted at specific genetic mutations linked to diseases. These technological developments establish siRNA as a robust and flexible instrument for immune system modulation which shows great promise to transform therapies for immune-based conditions.
References
- de Brito e Cunha, Danielle, et al. "Biotechnological evolution of siRNA molecules: from bench tool to the refined drug." Pharmaceuticals 15.5 (2022): 575. https://doi.org/10.3390/ph15050575.
- Sioud, Mouldy. "Releasing the immune system brakes using siRNAs enhances cancer immunotherapy." Cancers 11.2 (2019): 176. https://doi.org/10.3390/cancers11020176.
- Motamedi, Hamid, et al. "Principle, application and challenges of development siRNA-based therapeutics against bacterial and viral infections: a comprehensive review." Frontiers in Microbiology 15 (2024): 1393646. https://doi.org/10.3389/fmicb.2024.1393646.
- Ali Zaidi, Syed Saqib, et al. "Engineering siRNA therapeutics: challenges and strategies." Journal of Nanobiotechnology 21.1 (2023): 381. https://doi.org/10.1186/s12951-023-02147-z.
- Meng, Zhongji, and Mengji Lu. "RNA interference-induced innate immunity, off-target effect, or immune adjuvant?." Frontiers in immunology 8 (2017): 331. https://doi.org/10.3389/fimmu.2017.00331.
- Distributed under Open Access license CC BY 4.0, without modification.
