The RNAi Revolution-Rewriting the Rules of Genetic Medicine
What is RNA interference
RNA interference (RNAi) refers to the biological mechanism where double-stranded RNA triggers specific silencing of genes. The enzyme Dicer which resembles RNAse II generates small interfering RNAs (siRNAs) that measure between 21 and 23 nucleotides from long double-stranded RNAs to direct RNAi. siRNAs join the RNA-induced silencing complex (RISC) which recognizes and destroys matching mRNA sequences. Since its discovery in 1998 RNAi has demonstrated effectiveness across a range of organisms including trypanosomes, nematodes and vertebrates. Current research applications include studying gene function within living organisms and developing therapies for viruses and cancer as well as performing genome-wide screening. siRNA acts as an exact and adjustable mechanism to deactivate disease-related genes. siRNA functions by targeting mRNA and directly silencing harmful genes which leads to more precise therapeutic treatment options. The precise nature of siRNA enables it to function as an efficient treatment for various diseases including viral infections and cancer by targeting their genetic origins.
siRNA Mechanism
Mammalian cells generate siRNAs through RNaseIII endonuclease Dicer which cleaves longer dsRNA precursors. The TAR-RNA binding protein (TRBP) partners with Dicer to transfer siRNAs to the RNA-induced silencing complex (RISC) which includes Argonaute 2 to break target mRNA molecules between bases 10 and 11 from the 5' end of the antisense siRNA strand. The Argonaute (Ago) family members form the essential elements of RISC but only Ago-2 has the active catalytic domain that can perform cleavage in human cells. The double-stranded siRNAs get loaded into RISC but Ago-2 cleaves these to release the "passenger" strand which results in RISC activation with a single-stranded "guide" RNA molecule that directs target recognition through intermolecular base pairing. The rules for strand loading selectivity into RISC depend on the variable thermodynamic stability between the two ends of the siRNAs. Ago-2 binds to the PIWI domain via the guide strand whose 5' end unwinds from the less thermodynamically stable end. Ago-2 identifies and cuts messenger RNA molecules that show perfect or near-perfect match with the guide RNA.
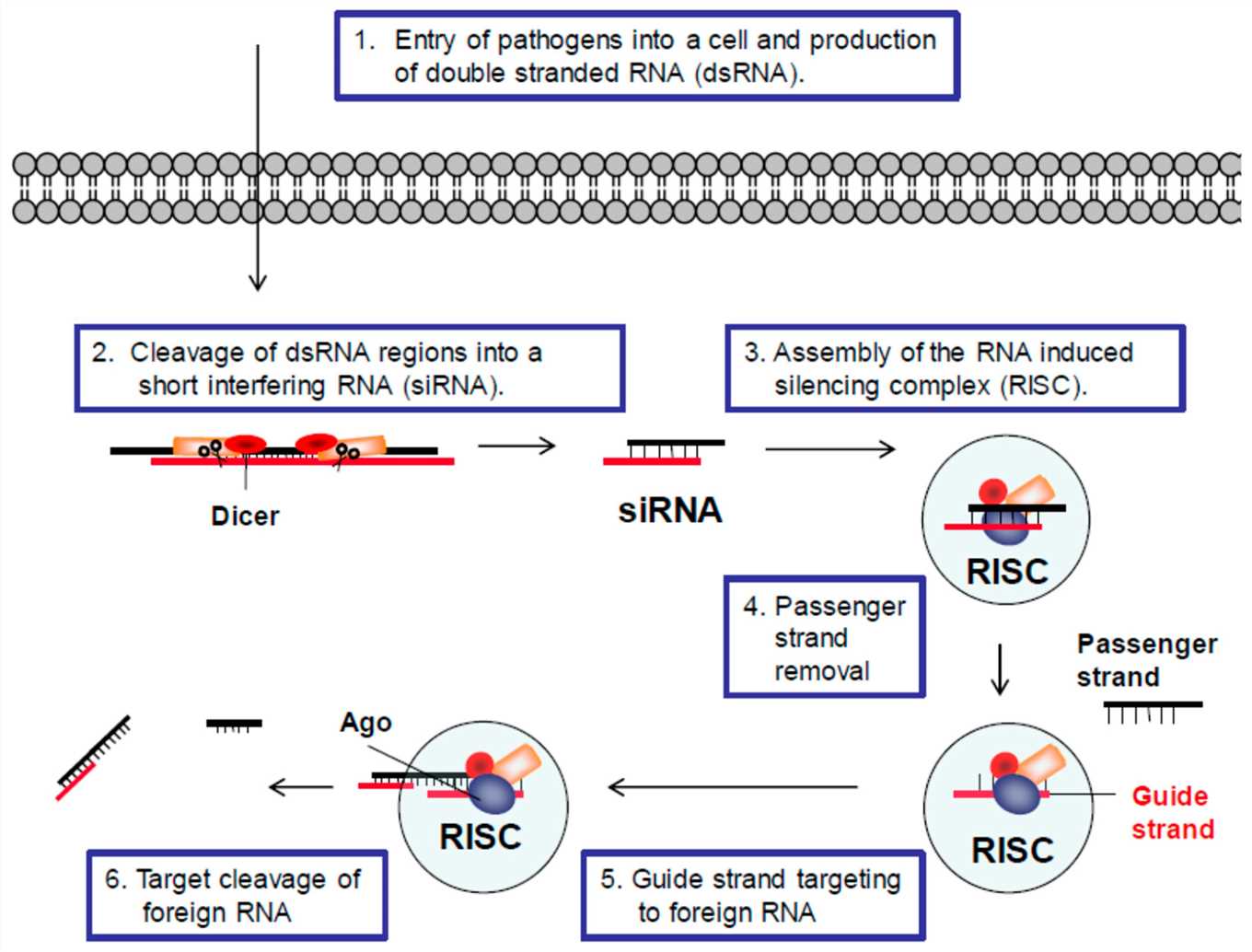 Fig.1 RNA interference (RNAi) defense pathway1,6.
Fig.1 RNA interference (RNAi) defense pathway1,6.
siRNA's Toolbox: Designing the Perfect Genetic Silencer
- Sequence Selection: Targeting the Right mRNA for Maximum Effect
Researchers must select siRNA sequences which bind exclusively to their target mRNA to prevent interactions with non-target genes. Computational software helps scientists identify mRNA regions that remain unpaired and have minimal sequence similarity to other genetic materials. The chosen sequences are chemically synthesized into siRNA molecules capable of targeting and degrading the mRNA.
- Chemical Modifications: Enhancing Stability and Reducing Off-Target Effects
Scientists enhance siRNA molecule stability and functionality through various chemical modification techniques. The siRNA backbone includes chemical additions such as 2'-O-methyl groups and locked nucleic acids (LNAs) which enhance its stability against degradation while minimizing off-target effects. The stability of siRNA molecules inside biological systems gets enhanced thanks to phosphorothioate linkages. Chemical modifications maintain siRNA activity while enabling precise targeting of mRNA molecules.
- Delivery Vehicles: LNPs, GalNAc Conjugates, and Viral Vectors
Scientists experience a major difficulty when they try to deliver siRNA molecules to their target cells. Lipid nanoparticles (LNPs) serve as delivery vehicles for siRNA because they shield siRNA from degradation and enable cellular uptake. Scientists employ N-acetylgalactosamine (GalNAc) conjugation with siRNA to improve its delivery to liver cells. Scientists employ adeno-associated viruses as viral vectors to administer siRNA for therapeutic interventions that require prolonged gene silencing.Target tissue delivery of siRNA depends on these specialized transport mechanisms to ensure successful delivery and function.
siRNA in Disease: A Precision Strike Force
- Oncology: Silencing Oncogenes
The G12D mutation accounts for the majority of KRAS alterations which plays a pivotal role in pancreatic cancer development. The use of siRNA to target and silence KRAS genes shows significant therapeutic promise. The biodegradable siG12D LODER polymer serves as a delivery system for KRASG12D-specific siRNA in tumor cells. Patients with KRASG12D or G12V mutations experienced longer overall survival when treated with siG12D LODER combined with chemotherapy based on clinical trial findings. Researchers developed a treatment approach using cRGD peptide-modified polymersomes which deliver siRNA against KRASG12D. Preclinical studies showed that pancreatic tumors displayed substantial tumor suppression and survival improvement when nanoparticles managed to reduce KRASG12D expression by 90%. Recent advancements establish that siRNA can accurately target oncogenic genes within pancreatic cancer.
- Metabolic Disorders: Lowering PCSK9 for Cholesterol Control
Inclisiran uses siRNA technology to target PCSK9, which regulates low-density lipoprotein cholesterol (LDL-C) levels. Inclisiran's PCSK9 silencing mechanism leads to lower LDL-C levels that decrease cardiovascular disease risk. Research demonstrates that biannual subcutaneous injections of inclisiran produce a 50% decrease in LDL-C levels. This new method presents substantial benefits compared to traditional statin treatments because their effectiveness varies and they frequently lead to adverse effects. The minimal dosing requirement and sustained LDL-C control presented by inclisiran make it a strong candidate for treating hypercholesterolemia.
- Rare Diseases: Treating Amyloidosis (Patisiran) and Porphyria
The siRNA-based medication patisiran has been approved to treat hereditary transthyretin-mediated amyloidosis which causes amyloid fibrils accumulation. Patisiran functions as an siRNA treatment that silences messenger RNA responsible for transthyretin protein production leading to reduced amyloidogenic protein formation. Acute hepatic porphyria represents another rare metabolic disorder which givosiran treats through siRNA therapy. The siRNA therapy givosiran achieves disease management by reducing toxic porphyrin precursor accumulation through the targeting of aminolevulinic acid synthase 1 (ALAS1). These examples demonstrate how siRNA technology can tackle the genetic roots of rare diseases.
- Infectious Diseases: siRNA's Role in Antiviral Therapies
siRNA demonstrates potential for antiviral treatments which includes combating the virus SARS-CoV-2 that causes COVID-19. Research before testing in humans shows that siRNA successfully blocks viral genes to stop both viral replication and its distribution. siRNA that targets the viral RNA-dependent RNA polymerase (RdRp) demonstrated antiviral effects in experimental cell cultures and animal studies. This method presents a quick pathway to create antiviral therapies particularly for newly discovered viruses. The targeted action of siRNA enables accurate identification of viral sequences while reducing both resistance risks and off-target effects.
Delivery Breakthroughs for siRNA
- Liver-First Success
The development of GalNAc-siRNA conjugates transformed hepatic siRNA delivery methods. Hepatocyte surface ASGPR receptors target GalNAc conjugates for binding. Through rapid endocytosis activation, the binding process enables siRNA to penetrate cells and initiate RNAi mechanisms. GalNAc-siRNA conjugates have become the top delivery method for liver-targeted disease treatments because of their straightforward application and superior effectiveness compared to lipid nanoparticle (LNP) systems. In 2019 the FDA gave its approval for givosiran which functions as a GalNAc-siRNA conjugate to treat acute hepatic porphyria. The development of GalNAc-conjugated siRNA therapies in clinical trials is now possible because a prior treatment success showed their effectiveness against various liver diseases.
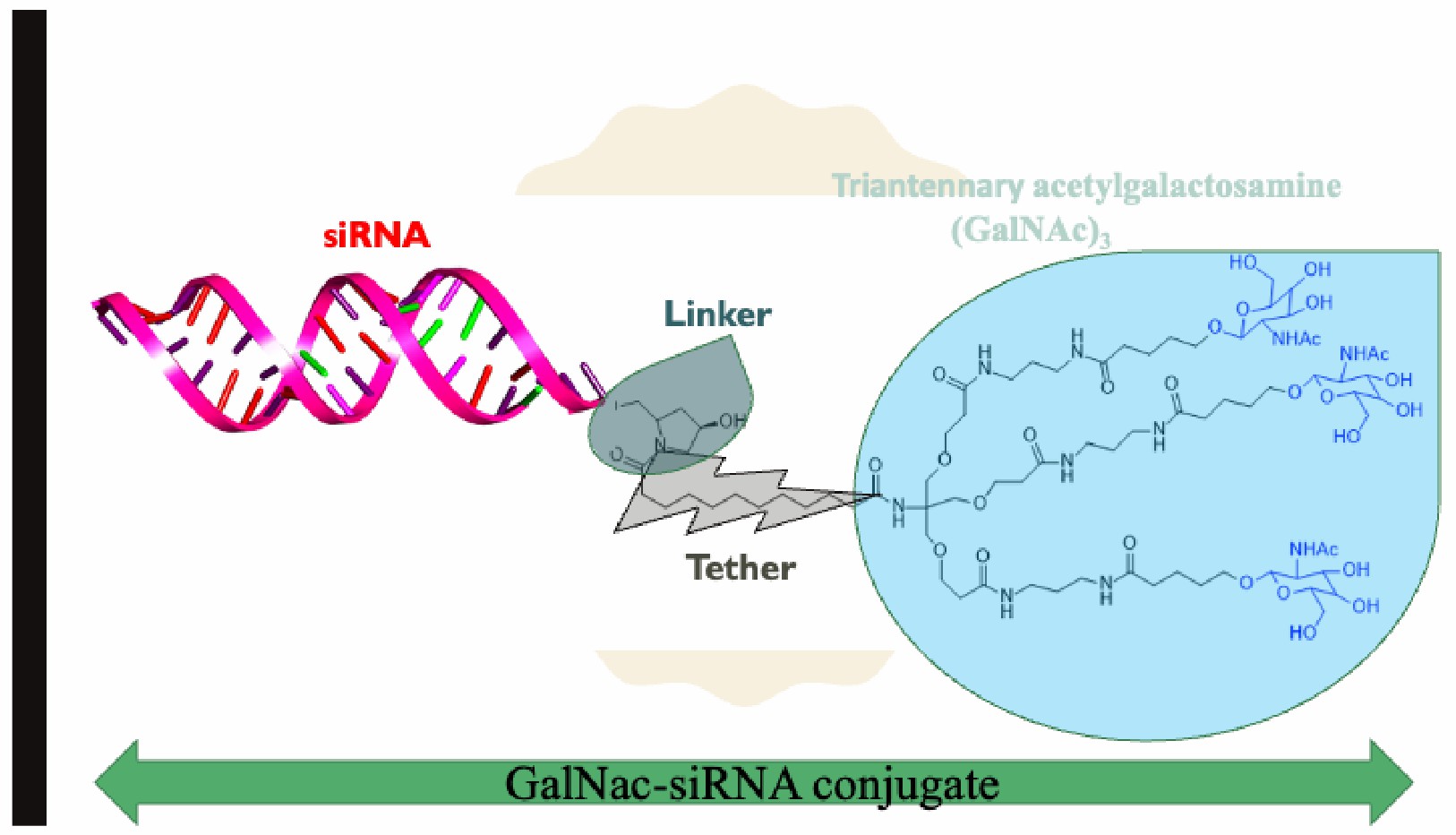 Fig.2 Chemical structure of triantennary acetylgalactosamine (GalNAc)3 hybridized with siRNA2,6.
Fig.2 Chemical structure of triantennary acetylgalactosamine (GalNAc)3 hybridized with siRNA2,6.
- Beyond the Liver: Targeting Brain, Heart, and Solid Tumors
Research advancements in siRNA delivery extend beyond liver applications. Scientists are investigating methods to apply siRNA delivery to various tissues beyond the liver like the brain, heart, and solid tumors. Researchers have demonstrated that focused ultrasound together with microbubble-assisted delivery successfully transports siRNA-loaded nanoparticles to brain tumor tissues and achieved more than ten times better delivery rates while substantially boosting tumor cell death. SiRNA therapy has been applied in heart disease treatments to turn off genes associated with inflammatory responses after heart attacks which lead to arrhythmias. siRNA directed against oncogenes such as KRAS demonstrates potential in preclinical studies of solid tumors when combined with novel delivery systems that improve siRNA uptake and effectiveness.
- Exosomes, Polymer Nanoparticles, and Cell-Penetrating Peptides
Scientists are developing siRNA delivery systems that utilize exosomes and polymer nanoparticles in combination with cell-penetrating peptides (CPPs). Exosomes can be engineered by scientists to carry tissue-targeting molecules on their surfaces which allows for precise siRNA delivery to specific tissues. Researchers have engineered exosomes containing rabies virus glycoprotein (RVG)-derived peptides for delivering siRNA into brain tissues. Scientists have developed a promising novel approach to siRNA delivery through polymer nanoparticles made with materials such as chitosan and hyaluronic acid. The nanoparticles shield siRNA from breakdown while helping target cells to absorb it. By conjugating CPPs to siRNA or presenting them on exosome surfaces scientists improve cellular entry and protect the negative siRNA charge which enhances delivery efficiency. Advanced delivery systems play a vital role in broadening siRNA therapy applications beyond liver treatment while overcoming obstacles in delivering siRNA to multiple tissue types.
siRNA's Unique Advantages Over Conventional Therapies
- Precision medicine
siRNA shows remarkable precision in gene targeting. Researchers design siRNA molecules to bind with a specific gene for targeted action. The siRNA sequence binds to a particular region of the target gene's mRNA based on base-pairing complementarity principles. siRNAs offer precise gene silencing of mutated or overexpressed genes in specific genetic diseases without affecting normal gene function. The high precision of this approach prevents unintended interactions and offers substantial benefits compared to traditional treatments. Traditional small - molecule drugs frequently bind to various proteins or receptors which creates opportunities for side effects. siRNA therapies provide targeted treatment which enhances therapeutic effectiveness while minimizing negative side effects.
- Long-lasting effects
The administration of one dose of siRNA results in gene silencing effects that extend over several months. The RNAi pathway serves as the mechanism through which siRNA operates. The siRNA enters the cell and becomes a part of the RISC. The RISC uses the attached siRNA to direct the breakdown of the target mRNA. The ability of siRNA to be recycled and reused enables ongoing degradation of the target mRNA throughout an extended timeframe. The durable gene silencing effect enables chronic disease treatment by decreasing how often drugs need to be administered. Long-term use of siRNA to turn off disease-related genes can decelerate disease progression in certain neurodegenerative disorders.
- Rapid development
The design process for siRNA is faster compared to small molecules or biologics. Scientists can quickly create the appropriate siRNA sequence for therapy by examining the target gene's nucleotide sequence once they have identified it. Chemical synthesis techniques keep siRNA synthesis straightforward and uncomplicated. Small molecule drug development demands thorough screening processes and chemical structure optimization to reach the targeted pharmacological effects. Manufacturing biologics including monoclonal antibodies requires intricate methods for protein expression and purification. siRNA-based therapies enable faster development processes which can reduce the period between laboratory research and clinical application for new treatments.
Beyond Therapy: siRNA as a Scientific and Diagnostic Tool
Researchers use siRNA extensively in studies to understand gene functions and to validate target genes. Basic research employs siRNA technology to suppress specific genes in laboratory cells and experimental organisms. Scientists determine the function of the target gene by studying phenotypic changes that occur after gene knockdown. In cancer research scientists use siRNAs to block genes that are presumed to play roles in tumor progression and metastasis. The approach enables researchers to discover potential therapeutic targets and study how cancer develops at the molecular level. Within drug discovery initiatives scientists utilize siRNA-mediated gene silencing to assess the validity of potential drug targets. When gene silencing results in a significant phenotypic change related to the disease it suggests that the gene is worth exploring as a drug development target. The detection of disease-specific RNA signatures is possible through the application of siRNA. Characteristic gene expression changes frequently accompany different disease types. Researchers can develop diagnostic assays by designing siRNAs that selectively target disease-associated RNAs. SiRNA design enables the creation of molecules that specifically target viral RNAs to facilitate viral infection diagnosis. Detection of viral RNA happens through observing how the siRNA silences the target RNA.
References
- Scarborough, Robert J., and Anne Gatignol. "RNA interference therapies for an HIV-1 functional cure." Viruses 10.1 (2017): 8. https://doi.org/10.3390/v10010008.
- Ebenezer, Oluwakemi, et al. "Development of novel siRNA therapeutics: a review with a focus on inclisiran for the treatment of hypercholesterolemia." International Journal of Molecular Sciences 24.4 (2023): 4019. https://doi.org/10.3390/ijms24044019.
- Sinning, David, and Ulf Landmesser. "Low-density lipoprotein-cholesterol lowering strategies for prevention of atherosclerotic cardiovascular disease: focus on siRNA treatment targeting PCSK9 (Inclisiran)." Current Cardiology Reports 22 (2020): 1-7. https://doi.org/10.1007/s11886-020-01427-6.
- Tian, Zhili, et al. "Insight into the prospects for RNAi therapy of cancer." Frontiers in Pharmacology 12 (2021): 644718. https://doi.org/10.3389/fphar.2021.644718.
- Ashrafizadeh, Milad, et al. "Progress in delivery of siRNA-based therapeutics employing nano-vehicles for treatment of prostate cancer." Bioengineering 7.3 (2020): 91. https://doi.org/10.3390/bioengineering7030091.
- Distributed under Open Access license CC BY 4.0, without modification.
