Metabolic Mayhem-siRNA's Strike Against Obesity and Diabetes
Introduction of siRNA in Metabolic Regulation
Small interfering RNA (siRNA) functions as a biological tool to silence genes through targeted mRNA degradation using the RNA interference (RNAi) pathway. Double-stranded RNA (dsRNA) is delivered to cells by scientists where the Dicer enzyme processes it into short siRNA fragments measuring between 21 and 23 nucleotides. The siRNA fragments integrate into the RNA-induced silencing complex (RISC) to guide these fragments toward matching mRNA sequences which results in mRNA destruction and blocks protein synthesis. RNAi functions as cells' natural defense system which targets and eliminates viral RNA and transposon sequences. The natural RNAi pathway provides a tool for therapeutic gene silencing to target disease-related genes. Scientists can engineer siRNA molecules to target mRNA sequences responsible for producing proteins which drive metabolic diseases like obesity and diabetes. Through targeted gene silencing using siRNA scientists can correct metabolic dysfunctions and produce better results in disease treatment.
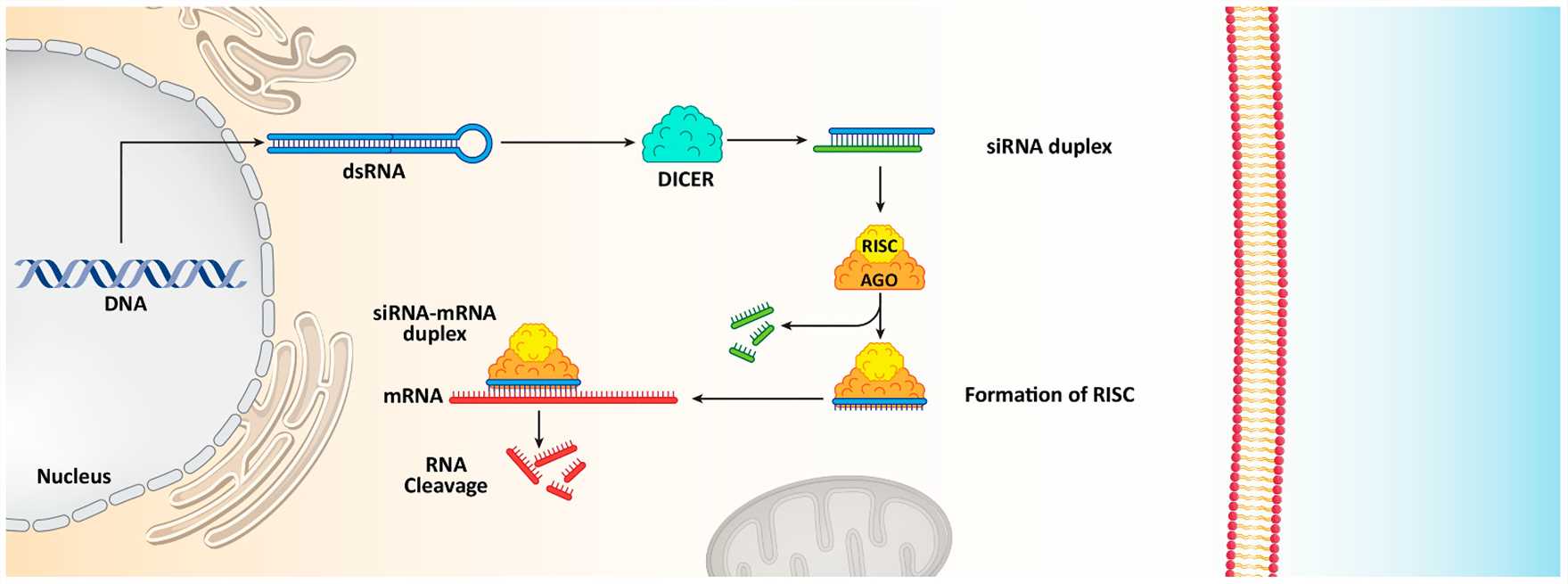 Fig. 1 The functions of siRNA in reducing gene expression and causing mRNA cleavage1,6.
Fig. 1 The functions of siRNA in reducing gene expression and causing mRNA cleavage1,6.
Introduction: The Global Metabolic Crisis
The worldwide metabolic crisis manifests through worrisome figures regarding obesity and diabetes prevalence. As of now, obesity affects more than 1 billion people across the globe while diabetes impacts over 500 million people. Healthcare systems and global economies will face a substantial burden because projected figures show major increases in these numbers throughout the upcoming decades. Obesity and diabetes treatments remain insufficient as they demand long-term medication use which produces extensive side effects. Bariatric surgery functions as an invasive treatment option which cannot be administered to every patient. These conventional treatments do not tackle the fundamental causes of metabolic disorders which results in unsatisfactory results and high chances of relapse. siRNA serves as a genetic scalpel that precisely targets and silences specific genes responsible for metabolic dysregulation and offers a promising treatment alternative. siRNA therapies target fundamental genetic and molecular factors of obesity and diabetes to create treatments that work better over time while producing few side effects. By correcting fundamental genetic factors this targeted method has the potential to transform metabolic disorder treatment strategies.
siRNA's Anti-Obesity Tactics: Silencing the Fat Triggers
- Target FASN (Fatty Acid Synthase)-Blocks Fat Production
Fatty acid synthase (FASN) operates as an essential enzyme in the de novo synthesis of fatty acids which becomes more active during obesity and related metabolic diseases. Inhibition of FASN successfully prevents fat production and decreases lipid buildup within tissue structures. Research has shown that siRNA directed at FASN can significantly decrease FASN expression and fat synthesis across in vitro and in vivo studies. Research on animal models shows that FASN siRNA therapy reduces body weight while improving metabolic profiles without affecting food consumption which indicates its promising application for treating obesity.
- Target PCSK9-Lowers Cholesterol
PCSK9 is a protein that controls cholesterol by causing low-density lipoprotein receptors (LDL-Rs) to degrade in the liver. When PCSK9 is inhibited, it results in increased LDL-R availability which lowers LDL cholesterol levels in the blood. Clinical trials demonstrate that siRNA agents like Inclisiran targeting PCSK9 achieve substantial LDL-C reduction. Inclisiran functions by selectively destroying PCSK9 mRNA which leads to decreased production of the PCSK9 protein. Patients receive injections every six months because this approach produces long-term effects. Research indicates Inclisiran lowers LDL-C by 50% which positions it as an effective treatment for hypercholesterolemia and cardiovascular risk reduction.
- Target Ghrelin Receptors-Curbs Hunger Signals
This hormone acts as a promoter for both appetite stimulation and fat storage accumulation. siRNA that targets ghrelin receptors diminishes hunger signals while lowering food consumption which may result in weight loss. Research using animal models demonstrated significant body weight reductions and metabolic improvements when ghrelin receptors were targeted by siRNA. This method presents a fresh approach to controlling appetite and managing body weight through direct intervention in hunger and fullness hormonal pathways.
- Case Study: Animal Trials Showing Weight Loss Without Diet Changes
Research with animals has proven that siRNA-based treatments can successfully induce weight loss without requiring dietary changes. Obese mice undergoing treatment with siRNA targeting FASN showed notable weight reduction and enhanced metabolic profiles when compared to control groups. Animal models treated with siRNA aimed at PCSK9 experienced lower cholesterol levels and better cardiovascular health outcomes. Research demonstrates that siRNA can serve as a powerful solution for obesity and related metabolic disorders by targeting essential metabolic pathways which reduces dependence on lifestyle modifications.
Preclinical applications of siRNA for Metabolic Regulation
- Target glucagon receptor (Gcgr)
Researchers in 2017 performed an experiment on mice predisposed to diabetes which received both a high-fat diet and streptozotocin (STZ) treatment to develop diabetes. Researchers conducted this study to examine how well a siRNA formulation worked. Lipid nanoparticles carried the therapeutic siRNA combination of leptin and glucagon-siRNA in this formulation. The experimental results demonstrated no notable impact on blood sugar levels from administering 5 mg/kg of Gcgr-siRNA. The application of Gcgr-siRNA at a dosage of 10 mg/kg produced a substantial increase in glucagon plasma concentrations along with a significant reduction in blood glucose levels. Three weeks of Gcgr-siRNA treatment at 10 mg/kg dosage resulted in both better oral glucose tolerance and lower blood glucose levels. Two months into treatment patients experienced lower blood glucose levels and improved oral glucose tolerance performance. The administration of leptin therapy resulted in the normalization of blood glucose levels along with oral glucose tolerance and plasma ketones while improving slow lipid metabolism. The treatment had no significant effect on the regulation of glucose homeostasis. The siRNA formulation aimed at Gcgr demonstrated positive effects in regulating blood glucose levels and improving oral glucose tolerance in diabetic mice. Leptin treatment failed to produce any effect on glucose homeostasis.
- Target IHoP
In 2017 researchers used siRNA to suppress IHoP expression and demonstrated that blocking this pancreatic α-cells protein in diabetic mice halted disease progression. The findings support the conclusion that IHoP has an important function in controlling glucagon secretion while also preserving glucose balance in the body. siRNA-based targeting of IHoP emerges as a promising treatment strategy for diabetes management. The study used siRNA to target the TORC2 gene for expression suppression while exploring its potential as a diabetes treatment strategy. The research findings indicated that the use of siRNA to suppress the TORC2 gene proved beneficial for diabetes treatment. Reduction of TORC2 activity modified the gluconeogenesis pathway leading to lower glucose release and improved glucose balance. Altering TORC2 activity presents an opportunity to improve blood glucose level management and advance diabetes treatment methods.
- Target Fas
Scientists used non-obese diabetic (NOD) mice to target and suppress Fas gene expression which plays a role in diabetes development. Scientists found that using PEI-based siRNA administration led to reduced Fas expression within the pancreas. The decrease in Fas gene expression delayed the start of insulitis that affects pancreatic beta cells while simultaneously speeding up the rise of cyclophosphamide-induced hyperglycemia in NOD mice. This study demonstrates the potential of targeting specific genes involved in diabetes pathogenesis by using siRNA to silence Fas gene expression while using PEI as a delivery carrier. The study indicates that altering Fas expression impacts autoimmune diabetes development in NOD mice which opens new avenues for disease management therapies.
Delivery Breakthroughs: Hitting the Right Tissues
Challenges: siRNA degradation, reaching fat/liver/pancreas
Ensuring that therapeutic siRNA molecules reach their target cells without degradation depends on the development of effective delivery systems. The highly anionic and hydrophilic nature of siRNA molecules prevents them from easily crossing the hydrophobic lipid bilayer of the plasma membrane through passive diffusion. siRNA gets quickly eliminated through the kidneys while its bloodstream presence lasts only 6 minutes to 1 hour. The development of delivery systems which shield siRNA from degradation while boosting cellular uptake and endosomal escape is required to address these challenges.
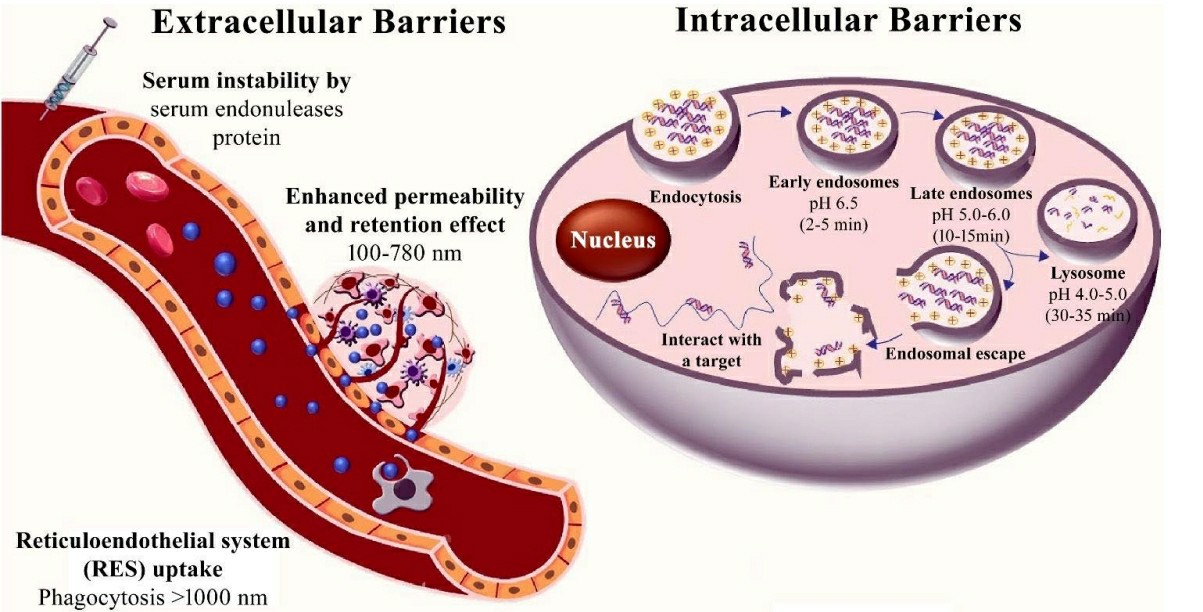 Fig. 2 Extracellular and intracellular barriers for siRNA therapeutics2,6.
Fig. 2 Extracellular and intracellular barriers for siRNA therapeutics2,6.
Strategies for enhance the Delivery of siRNA
- Lipid Nanoparticles
LNPs stand as a fundamental platform for siRNA delivery by improving both cellular uptake and molecular stability. The composition of LNPs includes lipids together with cholesterol and PEGylated lipids which creates a structure capable of encapsulating siRNA inside the core. By attaching targeting ligands to the nanoparticles' surface researchers can focus siRNA delivery on certain cell types which enhances gene silencing precision. Alnylam Pharmaceuticals created SNALPs (Stable Nucleic Acid-Lipid Particles) which enabled siRNA delivery to target VEGF and KSP in phase I clinical trials aimed at treating advanced solid tumors. The same technology has been used to transport PCSK9-targeting siRNA which effectively reduces cholesterol levels.
- Conjugated siRNAs
Conjugated siRNAs present an effective delivery method for targeted medical treatments. The N-acetylgalactosamine (GalNAc)-siRNA conjugate achieves high success rates because it forms strong connections with the asialoglycoprotein receptor (ASGPR) on hepatocyte surfaces. When siRNA targets the liver, it becomes highly effective against liver-related diseases. Alnylam Pharmaceuticals developed a trivalent GalNAc-siRNA delivery system to enhance liver targeting capabilities. Alnylam Pharmaceuticals has engineered a trivalent GalNAc-siRNA delivery system specifically to improve targeting of the liver. The siRNA drugs ALN-TTRsc, ALNPCS and ALN-AT3 have been developed by combining trivalent GalNAc with siRNAs that target TTR, PCSK9 and AT molecules respectively. Clinical trials have begun for these drugs which target thyrotropin amyloidosis along with hypercholesterolemia and hemophilia.
- Oral/Inhaled Formulations in Development
Researchers are currently developing oral and inhaled siRNA formulations as alternatives to injectable versions. The delivery methods focus on overcoming systemic administration difficulties while enhancing patient adherence. Scientists are investigating lipid-based nanoparticles and polymer-based formulations for oral siRNA delivery to protect it from gastrointestinal breakdown and improve its absorption rate. Researchers are developing inhaled formulations to deliver siRNA to lung epithelial cells as a treatment for lung diseases.
Advantages of siRNA Over Traditional Therapies
- Long-Lasting Effects
siRNA therapy stands out because it provides long-lasting therapeutic benefits. The long-term therapeutic benefits of siRNA therapies arise from their ability to sustain effectiveness after a single administration for several months. siRNA molecules sustain their therapeutic effects because they demonstrate inherent stability and a slow rate of degradation within the body. The siRNA medication patisiran reaches patients with hereditary transthyretin amyloidosis through a dosage schedule of one administration every three months. Longer-lasting therapeutic effects eliminate frequent dosing requirements and result in better treatment protocol adherence for patients.
- Precision: Fewer Side Effects Than Systemic Drugs
siRNA therapy selectively binds to mRNA from specific genes which allows it to disable deleterious genes without disturbing normal cell activities. Target precision in siRNA drugs decreases side effects compared to traditional systemic medications that broadly disrupt cellular pathways and harm healthy tissues. Engineered siRNA molecules can selectively turn off mutant gene versions that cause diseases like cancer and neurodegenerative disorders without affecting normal gene functions. The precise targeting strategy helps to reduce unwanted side effects and decrease adverse reactions.
- Potential to Combine Targets
The versatility of siRNA therapy lies in its ability to target multiple genes at once for effective treatment of complex diseases operating through various biological pathways. Scientists have the ability to design siRNA molecules that attack genes responsible for obesity and those linked to diabetes and cardiovascular disease (CVD) at the same time. The ability to multiplex enables the creation of combination therapies which tackle several disease factors at once. In metabolic disorders siRNA enables comprehensive treatment by silencing genes responsible for fat production like FASN, cholesterol regulation such as PCSK9 and appetite control through ghrelin receptors. Utilizing this method enables clinicians to decrease dependency on various medications while enhancing patient treatment results.
References
- Mirzaei, Sepideh, et al. "Pre-clinical and clinical applications of small interfering RNAs (siRNA) and co-delivery systems for pancreatic cancer therapy." Cells 10.12 (2021): 3348. https://doi.org/10.3390/cells10123348.
- Ali Zaidi, Syed Saqib, et al. "Engineering siRNA therapeutics: challenges and strategies." Journal of Nanobiotechnology 21.1 (2023): 381. https://doi.org/10.1186/s12951-023-02147-z.
- Forte, Yasmin Silva, Mariana Renovato-Martins, and Christina Barja-Fidalgo. "Cellular and molecular mechanisms associating obesity to bone loss." Cells 12.4 (2023): 521. https://doi.org/10.3390/cells12040521.
- Golounina, Olga, et al. "Pathogenetic therapeutic approaches for endocrine diseases based on antisense oligonucleotides and RNA-interference." Frontiers in Endocrinology 16 (2025): 1525373. https://doi.org/10.3389/fendo.2025.1525373.
- Zhang, Yanzhen, et al. "Inclisiran: a new generation of lipid-lowering siRNA therapeutic." Frontiers in Pharmacology 14 (2023): 1260921. https://doi.org/10.3389/fphar.2023.1260921.
- Distributed under Open Access license CC BY 4.0, without modification.
