Beyond Silencing-The Expanding Universe of RNAi Applications
The evolution of siRNA
siRNA was initially a lab curiosity. The phenomenon of RNA interference (RNAi) was first identified in 1998 when researchers successfully inhibited a gene in C. elegans using double-stranded RNA (dsRNA) injections. Researchers managed to silence mammalian cell genes with synthetic siRNA in 2001 while also understanding the structural and operational principles of siRNA. The discoveries provided the essential groundwork needed to develop siRNA-based therapies. SiRNA has transitioned from basic laboratory research to clinical applications over time. The FDA approval of patisiran in 2018 established siRNA-based therapy as a clinical reality by transforming siRNA from a research instrument into a therapeutic juggernaut. The breakthrough discovery enabled scientists to embark on a new period of gene regulation and clinical treatment development. siRNA molecules are employed by the RNAi mechanism to destroy specific mRNA sequences which results in the suppression of gene expression. Through its application to diverse medical conditions including cancer and genetic disorders this precise and powerful tool has moved beyond basic research into clinical practice.
siRNA function: Next-Generation RNAi Therapeutics
- Self-delivering siRNA: Breaking through tissue barriers
Traditional siRNA therapy needs carriers to shield siRNA from degradation while supporting its entry into target cells. Self - delivering siRNA provides a means to penetrate tissue barriers without requiring extra carrier molecules. siRNA receives chemical modifications or it attaches to specific ligands to achieve this process. Chemical modifications can improve siRNA stability and membrane permeability which allows it to enter cells directly. The ligand-conjugated siRNA binds precisely to cellular receptors while being taken up inside target cells through the process of receptor-mediated endocytosis. This technology streamlines siRNA delivery while minimizing carrier-related toxicity and immunogenicity.
- Conditional siRNA: Smart triggers for tissue-specific activation
The function of conditional siRNA enables activation only within targeted tissue areas. Specific enzymes together with pH values and light serve as triggers for regulating this mechanism within designated tissues or cells. Certain siRNAs have been engineered to activate through enzymes predominant in tumor tissues. These siRNAs stay inactive in healthy tissues but become activated within tumor environments to silence genes associated with cancer. The described approach enhances the specificity and safety of siRNA therapies by minimizing off-target effects and potential side effects.
- Multiplexed siRNA: Simultaneous targeting of multiple disease pathways
Multiplexed siRNA technology allows for the simultaneous silencing of multiple genes that contribute to disease progression. Using a mixture of different siRNAs allows researchers to target numerous essential molecules and pathways within a disease process. Multiplexed siRNA technology allows concurrent targeting of cancer-related oncogenes, tumor suppressor genes as well as genes that drive tumor angiogenesis and metastasis. This comprehensive targeting strategy proves more effective in treating complex diseases than single-gene targeting because it can disrupt multiple parts of the disease-related network while enhancing therapeutic outcomes.
siRNA's Role in Vaccinology
- siRNA-vaccine hybrids: Combining gene silencing with immunization
siRNA-vaccine hybrids bring together gene silencing techniques with established immunization methods to create a powerful new approach. The hybrid method utilizes siRNA's ability to specifically silence genes that contribute to viral replication or immune system evasion thus increasing vaccine effectiveness. Scientists can create siRNA to target critical viral genes responsible for entry or replication which makes the virus more vulnerable to vaccine-induced immune responses. Researchers are developing next-generation COVID-19 vaccines using siRNA-vaccine hybrids as a promising new technology. Scientific studies have investigated how siRNA can be used to selectively attack the SARS-CoV-2 spike protein which now forms the cornerstone of existing mRNA vaccine technology. This hybrid vaccine merges siRNA and spike protein mRNA to both block viral gene expression and stimulate a strong immune response which may provide superior defense against new virus variants.
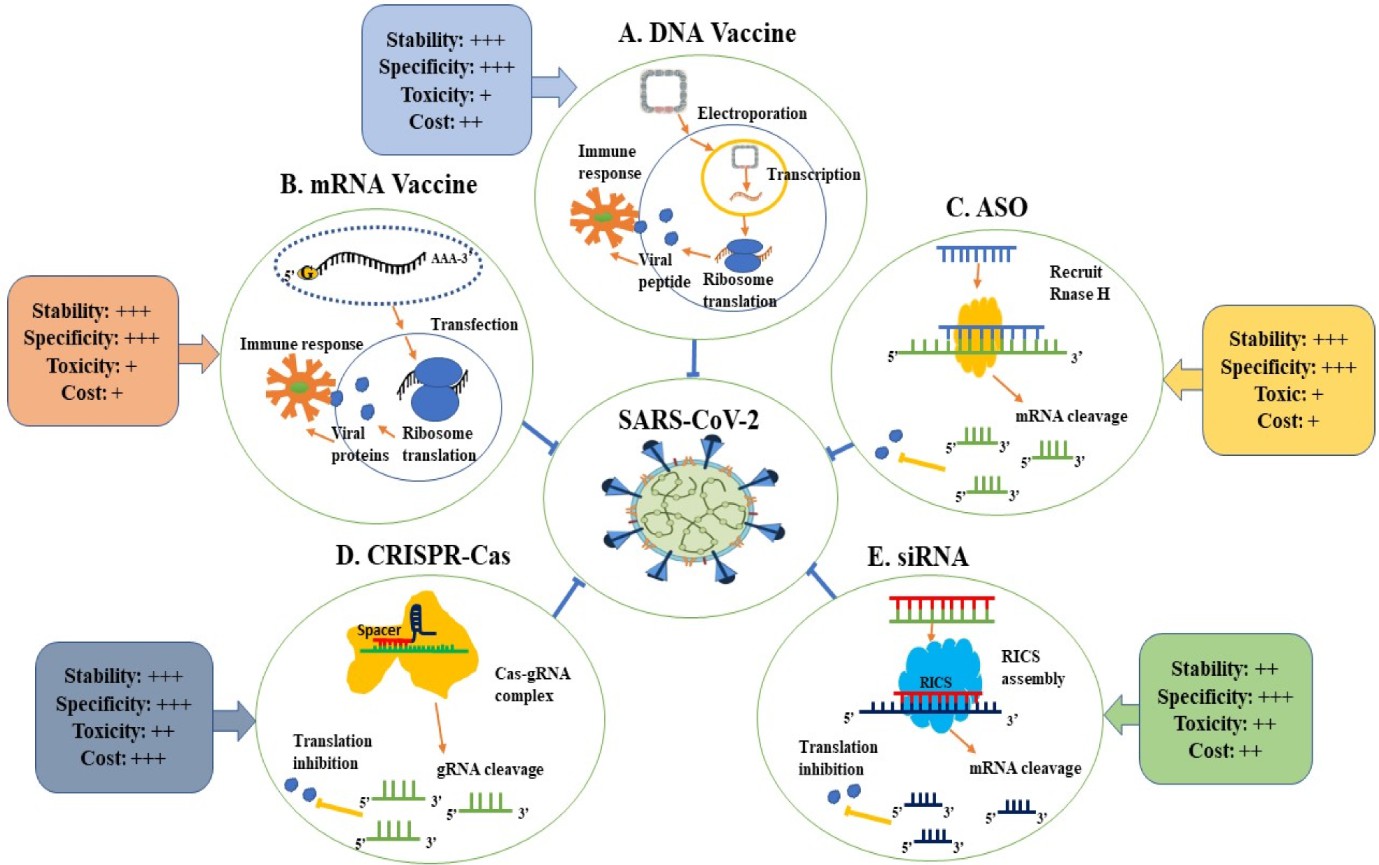 Fig.1 Applications of therapeutic nucleic acids in the fight against SARS-CoV-21,7.
Fig.1 Applications of therapeutic nucleic acids in the fight against SARS-CoV-21,7.
- Viral escape prevention: Using siRNA as an antiviral booster
A significant obstacle in creating antiviral treatments and vaccines stems from viral mutations which enable them to evade detection by the immune system. siRNA provides a robust antiviral strategy by attacking stable regions of the virus genome which viruses cannot easily change without losing functional strength. By focusing on conserved viral genome regions this method prevents escape mutations while improving treatment longevity. Multiple conserved regions of the SARS-CoV-2 genome such as the RNA-dependent RNA polymerase (RdRp) and the nucleocapsid (N) protein have been targeted using siRNA in COVID-19 research efforts. siRNA achieves broad-spectrum antiviral activity by targeting multiple conserved regions at once which decreases the potential for viral escape.
- Case study: siRNA-enhanced COVID-19 vaccine prototypes
One significant research example in siRNA-based COVID-19 vaccine development includes targeting the SARS-CoV-2 spike protein with siRNA. Research findings indicate that using siRNA together with mRNA vaccines improves immune responses and strengthens protection against variants of the virus. The hybrid technique suppresses essential viral genes and enhances vaccine effectiveness through improved immune system durability and strength. Researchers encapsulated siRNA within lipid nanoparticles to deliver it together with mRNA vaccines in one particular study. The siRNA directed its action towards the spike protein which led to decreased viral replication while simultaneously boosting vaccine effectiveness. The application of siRNA has displayed promising results in preclinical models which indicate its potential as an effective means to improve vaccine performance.
The Epigenetic Connection: siRNA's Hidden Talents
- siRNA-Directed DNA Methylation: Indirect Genome Editing
Scientific evidence shows that siRNA acts as a critical component in controlling DNA methylation which is an essential epigenetic modification leading to lasting changes in gene expression. The RdDM pathway in plants employs siRNAs to direct DNA methyltransferases toward specific genomic sites for new cytosine methylation. This mechanism maintains conservation across multiple eukaryotes and plays a critical role in transposon suppression along with gene imprinting processes. Similar regulatory systems featuring siRNAs and Argonaute proteins operate in mammals to control chromatin states but their exact workings remain poorly understood. The capability of siRNA to initiate DNA methylation suggests its use in indirect genome editing along with permanent gene silencing.
- siRNA Influences Gene Expression for Long-Term
Current research indicates that siRNA-mediated chromatin remodeling represents an emerging field because siRNAs can modify chromatin structure and thus control gene expression. Research findings indicate that siRNAs can guide histone-modifying enzymes to specific genomic sites which result in alterations to histone methylation and acetylation patterns. Research demonstrates that siRNAs can trigger histone H3K9 dimethylation which functions as a transcriptional repression signal. Through chromatin accessibility modifications this mechanism produces enduring gene expression alterations by affecting transcriptional machinery access. Multiple scientific studies have created models that demonstrate the dynamic relationship between siRNAs and chromatin states.
- Transgenerational Effects: Emerging Evidence in Model Organisms
Researchers have investigated siRNA's ability to produce transgenerational effects through experiments with different model organisms. siRNA-induced epigenetic alterations in plants can be passed down through generations and affect gene expression as well as stress response traits. Research shows that Schizosaccharomyces pombe, a lower eukaryote organism, utilizes siRNAs to create heterochromatin and silence repetitive sequences. Research indicates that epigenetic modifications caused by siRNA persist through generations, creating a means for traits to be inherited without genetic alterations. The current research indicates siRNA's capability to alter gene expression and phenotypes across generations although scientists are still exploring how these effects extend into mammals.
siRNA in the Brain: Conquering Neuroscience's Final Frontier
- Blood-Brain Barrier Breakthroughs: Novel Delivery Strategies
The blood-brain barrier (BBB) presents a major obstacle to siRNA delivery because of its selective permeability features. New delivery strategies demonstrate potential for overcoming this challenge. Researchers use intraventricular, intracranial and intranasal delivery methods to bypass systemic obstacles. Scientists delivered siRNA against BACE1 and APP through the intraventricular route by using copolymer-based micellar nanoparticles which consist of linear polyethyleneimine (LPEI)-g-polyethylene glycol (PEG). The infusion of the treatment method into the mouse brain’s lateral ventricles resulted in effective suppression of BACE1 and APP gene expression.
The nose-to-brain delivery of BACE1 siRNA is achieved through the application of solid lipid nanoparticles (SLNs). Studies demonstrate that both coated and uncoated solid lipid nanoparticles (SLNs) improve epithelial cell penetration which leads to better delivery of siRNA into the brain. The intranasal use of BACE1 siRNA with SLNs demonstrated promising results by improving cognitive abilities in transgenic mice models of Alzheimer's disease.
- siRNA's Potential Against Alzheimer's and Parkinson's
The potential of siRNA as a therapeutic solution for neurodegenerative diseases such as Alzheimer disease (AD) and Parkinson disease(PD) has been confirmed by scientific research. Research demonstrates that siRNA methods focused on BACE1 and APP represent a potential treatment for AD by reducing amyloid plaque formation and improving cognitive function. AD mouse models showed improved cognitive abilities after receiving intracranial injections of PEG-PEI/ROCK-II siRNA. BACE1 siRNA delivered intranasally using multifunctional siRNA nanocarriers enabled cognitive improvement in transgenic Alzheimer's disease mice.
Treatment with siRNA targeting α-synuclein shows promise in Parkinson's disease because it lowers levels of this disease-specific protein. Research demonstrates that neuronal cells show reduced α-synuclein levels while animal models exhibit improved motor performance when anionic liposomes with rabies virus glycoprotein-derived peptides are administered. The delivery of siRNA against α-synuclein using viral vectors and nanoparticles demonstrated substantial decreases in both protein levels and behavioral deficits.
- Psychiatric Applications: Silencing Addiction-Related Genes
siRNA demonstrates potential within psychiatric treatments by silencing genes that contribute to addiction. Researchers tested siRNA designed to target genes in the reward pathway related to addiction such as Drd2 (dopamine receptor D2) to explore potential treatments for addiction. Animal studies showed that genetic silencing of Drd2 in the nucleus accumbens using siRNA decreased cocaine-seeking behavior. Targeted gene expression modulation forms an innovative method to tackle addiction by precisely controlling the activity of essential addiction-related genes.
Agricultural Revolution: siRNA on the Farm
- Crop protection: Environmentally friendly pest control
Agricultural pest management now uses siRNA as a powerful environmentally sustainable method. Traditional chemical pesticides display broad-spectrum properties which damage helpful insects and result in environmental pollution. SiRNA-based pest control achieves high specificity by targeting essential survival genes of pest species exclusively. The precise nature of siRNA-based pest control protects non-target organisms while reducing environmental damage. Researchers created an RNA spray technique which precisely targets aphid genes resulting in elevated death rates and smaller populations. The method requires researchers to locate critical pest genome genes and create siRNA molecules that silence these genes. Researchers create a formulation that protects siRNA from environmental degradation before applying it directly to the target plants. The ingestion of treated plants by pests allows siRNA to enter their cells and initiate the RNAi pathway which results in target mRNA degradation and pest death.
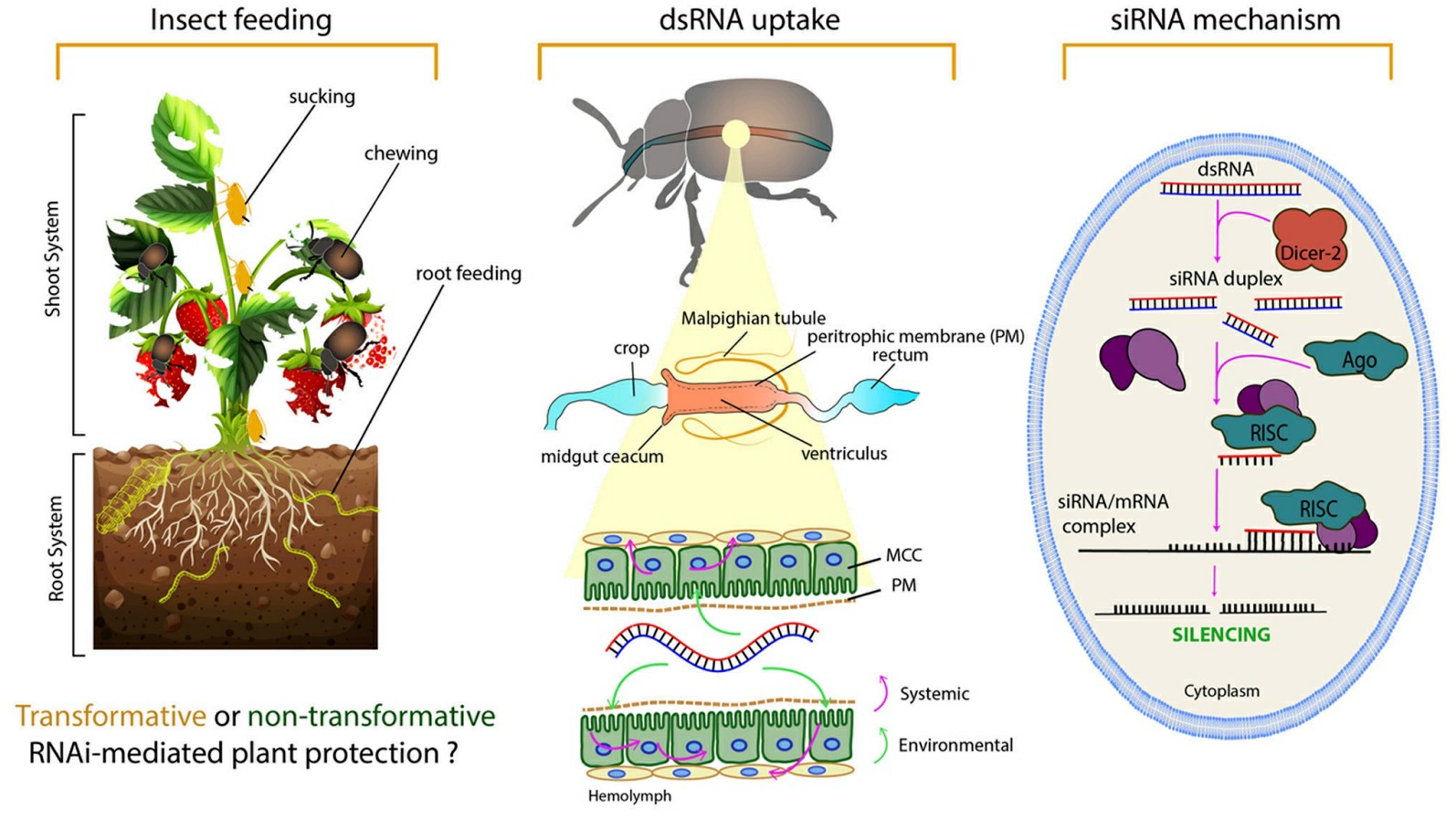 Fig.2 The basic levels of RNAi from an insect control perspective2,7.
Fig.2 The basic levels of RNAi from an insect control perspective2,7.
- Gene drives: siRNA-based population control of invasive species
The use of siRNA technology presents opportunities to manage invasive species populations via gene drive systems. These genetic elements known as gene drives enable rapid transmission through populations beyond the speed allowed by standard Mendelian inheritance. Researchers have the ability to use siRNA sequences within gene drives to suppress essential genes for invasive species survival and reproduction. Scientists can reduce fertility or increase mortality in invasive species populations by introducing siRNA that targets reproductive or developmental genes. This technique proves especially efficient against pest populations which have become resistant to conventional control methods.
- Edible siRNA: The future of genetically modified foods
Edible siRNA technology integrates siRNA molecules into agricultural crops to deliver health benefits or nutritional improvements. This method utilizes siRNA's capability to produce precise changes in gene expression. Researchers can create siRNA sequences that target genes which produce toxins while simultaneously boosting the expression of genes that generate beneficial nutrients. Scientists could develop genetically modified crops capable of silencing genes responsible for producing allergens and toxins. The reduction of gene expression through siRNA application leads to enhanced food safety in crops and decreased chances of allergic reactions.
siRNA as a Diagnostic Tool
- Living biosensors: siRNA circuits that detect disease markers
Research demonstrates that siRNA holds substantial potential for creating living biosensors capable of detecting disease markers with precise specificity and sensitivity. The RNA interference (RNAi) mechanism enables these biosensors to target specific mRNA sequences for degradation which facilitates biomarker detection related to different diseases. Researchers can create siRNA circuits that identify and react to specific viral RNA sequences to enable the early detection of diseases like COVID-19. The method offers a quick diagnostic solution that remains accurate alongside reduced occurrences of false results to improve disease detection reliability.
- Theranostic combos: Diagnosis and treatment in one package
The combination of diagnostic and therapeutic functions in one system shows great potential for siRNA technology through theranostic applications. The combined use of siRNA-based diagnostic circuits with therapeutic modules enables these systems to both recognize disease markers and administer targeted treatments at the same time. Engineered siRNA systems can release therapeutic substances when they identify specific biomarkers, thus delivering treatment exclusively at necessary times and locations. The dual functionality improves disease management precision while reducing systemic treatment side effects.
- Early warning systems: siRNA-based cancer detection
The innovative siRNA-based early warning systems detect cancer by using siRNA to target and eliminate specific oncogenic mRNA sequences. Monitoring cancer-related gene expression levels allows these systems to detect tumor development early and facilitate prompt treatment which leads to better patient results. Researchers can create siRNA circuits that identify when oncogenes like KRAS or MYC become overexpressed and then activate therapeutic siRNA molecules to silence these specific genes. This precise method improves the accuracy of cancer detection while offering early-stage tumor growth inhibition which may eliminate the necessity for invasive treatments.
References
- Abusalah, Mai Abdel Haleem, et al. "Nucleic acid-based COVID-19 therapy targeting cytokine storms: strategies to quell the storm." Journal of personalized medicine 12.3 (2022): 386. https://doi.org/10.3390/jpm12030386.
- Joga, Mallikarjuna R., et al. "RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: what we know so far." Frontiers in physiology 7 (2016): 553. https://doi.org/10.3389/fphys.2016.00553.
- Friedrich, Maik, et al. "Selection and validation of siRNAs preventing uptake and replication of SARS-CoV-2." Frontiers in bioengineering and biotechnology 10 (2022): 801870. https://doi.org/10.3389/fbioe.2022.801870.
- Wu, Renfei, and Kathy Qian Luo. "Developing effective siRNAs to reduce the expression of key viral genes of COVID-19." International Journal of Biological Sciences 17.6 (2021): 1521. https://www.ijbs.com/v17p1521.htm.
- Das, Protiva Rani, and Sherif M. Sherif. "Application of exogenous dsRNAs-induced RNAi in agriculture: challenges and triumphs." Frontiers in Plant Science 11 (2020): 946. https://doi.org/10.3389/fpls.2020.00946.
- Goyal, Rajat, et al. "Insights on prospects of nano-siRNA based approaches in treatment of Cancer." Frontiers in pharmacology 13 (2022): 985670. https://doi.org/10.3389/fphar.2022.985670.
- Distributed under Open Access license CC BY 4.0, without modification.
