siRNA in the Eye-Visionary Gene Silencing
Introduction to siRNA in Eye
Small interfering RNA (siRNA) offers treatment possibilities for eye diseases through RNA interference (RNAi) techniques that silence the genes connected to these disorders. Small interfering RNA molecules between 18 and 25 nucleotides long initiate post-transcriptional gene silencing mechanisms specific to sequences which enables precise regulation of pathways related to diseases.
Introduction to Ophthalmic Diseases
- Overview of Age-Related Macular Degeneration (AMD) and Glaucoma
AMD functions as the leading reason for vision loss in older adults since it leads to macular degeneration located at the retina's center responsible for sharp vision. The AMD pathophysiological process leads to photoreceptor cell death through the accumulation of drusen and persistent oxidative stress and inflammation. Glaucoma includes various eye disorders that result in optic nerve damage primarily because of increased intraocular pressure. Glaucoma emerges when aqueous humor production exceeds drainage capabilities resulting in optic nerve damage that causes vision loss which serves as the main risk factor for this condition. AMD treatment includes anti-VEGF therapies which prevent abnormal blood vessel development by blocking the vascular endothelial growth factor (VEGF). These treatments require regular intravitreal injections which create a heavy burden for patients. Glaucoma treatment fundamentally involves reducing intraocular pressure (IOP) through medication application as well as surgical and laser procedures. Existing treatments for AMD and glaucoma provide insufficient solutions for all patients along with significant side effects.
- siRNA as a Therapeutic Approach
The therapeutic approach of siRNA functions through gene silencing to target genes that cause AMD and glaucoma. Studies show that VEGF-targeted siRNA diminishes choroidal neovascularization in AMD research models. Targeted silencing of β-adrenergic receptor 2 (β2-AR) with siRNA leads to significant intraocular pressure reduction in glaucoma patients. These methods aim to minimize injection frequency while enhancing treatment effectiveness. Through the RNAi pathway siRNA guides RNA-induced silencing complexes (RISC) to eliminate target mRNA molecules. The mechanism enables exact gene silencing which decreases the production of harmful proteins. Lipid nanoparticles (LNPs) and viral vectors serve as delivery systems for siRNA therapies to maintain stability and uptake in ocular tissues. The siRNA SYL040012 known as Bamosiran targets β2-AR and achieved meaningful IOP reductions in animal models with glaucoma.
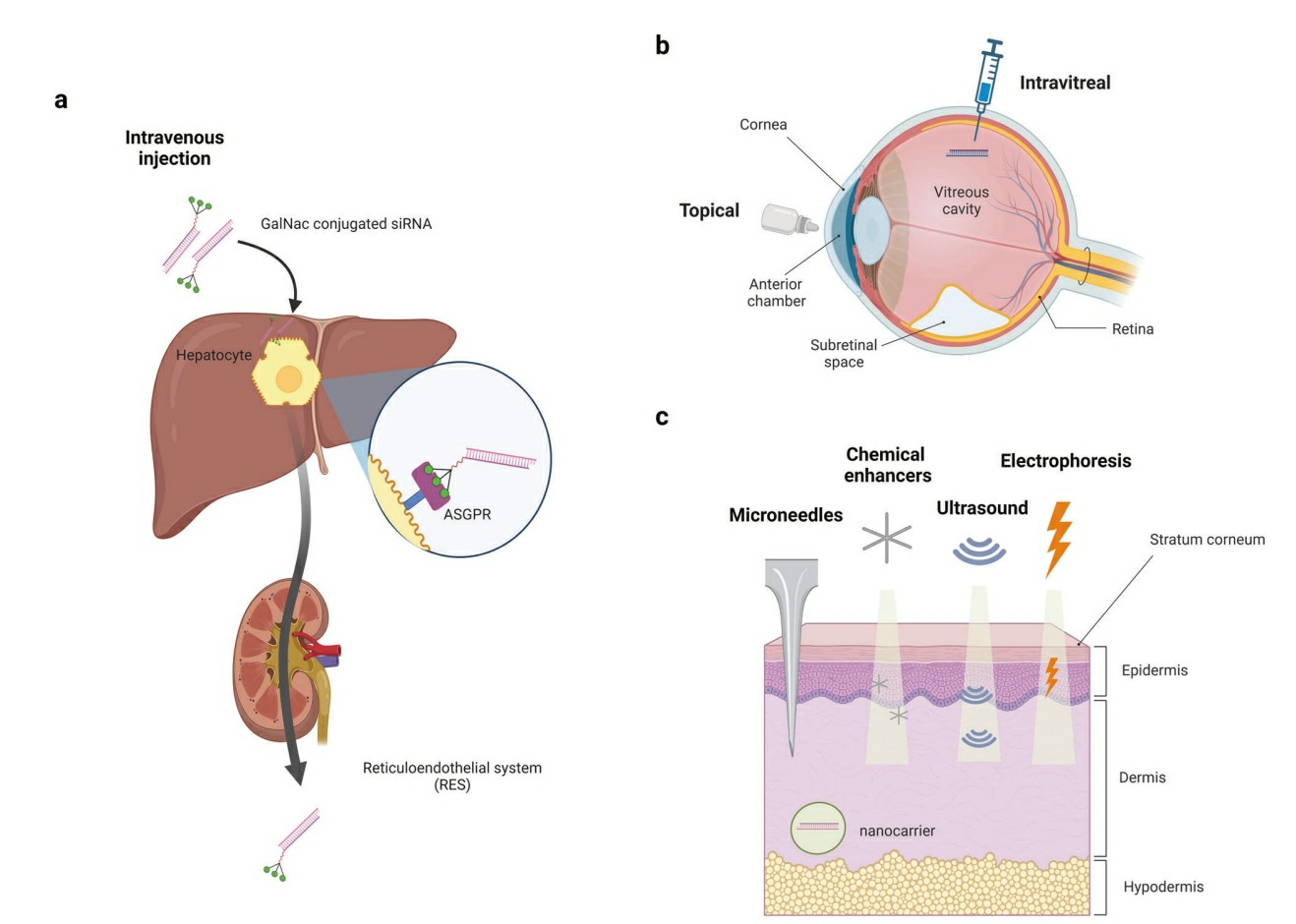 Fig.1 The characteristics of the liver, eye, and skin as target organs of siRNA drugs are described1,6.
Fig.1 The characteristics of the liver, eye, and skin as target organs of siRNA drugs are described1,6.
Mechanism of RNA interference in ocular tissues
The essential double-strand RNA (dsRNA) molecules known as siRNA participate intricately within the RNAi pathway. Cells rely on a complex mechanism to ensure precise gene regulation coordination. The endoribonuclease enzyme Dicer processes lengthier dsRNA or short hairpin RNAs to produce mature siRNA. This intricate process leads to the formation of siRNA which becomes a powerful tool for gene modulation. Synthetically-made siRNA which is artificial can reach cells directly to fulfill its specific purpose. Within cells siRNA associates with the RISC complex which contains essential proteins such as Dicer and Argonaute-2 (Ago-2). The guide antisense strand of siRNA stays in place following the removal of the passenger sense strand in response to activation stimuli. The guide strands attach themselves to messenger RNA segments that share complementary sequences. The Argonaute-2 protein within RISC executes a precise cleavage of mRNA which results in gene inactivation. Once the siRNA-loaded RISC completes its task with one mRNA molecule it can separate from it and attach to a new target mRNA to continue silencing multiple genes. SiRNA functions to silence genes during complex nucleic acid interactions.
siRNA in Ocular Biology
- Targeting Genes Involved in Retinal Degeneration
VEGF serves as a critical factor that drives the development of ocular neovascularization which occurs in AMD. VEGF triggers irregular blood vessel development in the retina which causes leakage and subsequent vision loss. The degeneration of the macula in AMD occurs due to oxidative stress along with the build-up of drusen within this central region of the retina that provides sharp vision. Researchers can create siRNA molecules to specifically destroy VEGF mRNA which results in decreased expression of VEGF and blocks the formation of new blood vessels. Research demonstrates that siRNA which targets VEGF achieves substantial CNV reduction in mouse studies. A research study showed that siRNA when delivered via bioreducible lipidoid nanoparticles successfully blocked retinal neovascularization in OIR rodent models while decreasing both VEGF mRNA and protein levels. The targeted method presents an alternative solution to existing anti-VEGF treatments that involve regular intravitreal injections.
- Inhibition of Inflammatory Pathways in the Eye
Through siRNA treatment scientists can turn off pro-inflammatory cytokines such as TNF-alpha that contribute to retinal inflammation and disease progression. Retinal inflammation serves as the main factor driving the onset of AMD and similar retinal degenerative conditions. Using siRNA to suppress cytokine expression can reduce inflammation and delay disease progression. Preclinical research shows siRNA that targets inflammation pathways exhibits potential therapeutic capabilities. The research demonstrated that siRNA that targeted TNF-alpha led to reduced retinal inflammation and enhanced retinal function in mice with signs of AMD pathology. This medical strategy tackles disease inflammation while showing potential to maintain retinal structure and function. Treatment efficacy could show additional improvements when siRNA is used alongside therapeutic agents like antioxidants.
Challenges limiting siRNA efficiency
- Off-target effects
Researchers have identified three separate categories of off-target effects concerning RNA interference. Saturation of the cellular RNA interference machinery causes miRNA-regulated genes to become upregulated in the first category of off-target effects. One possible solution to this problem involves using chemically modified siRNAs or selecting stronger siRNAs. The second category involves gene silencing that occurs through mechanisms associated with the passenger strand. The mechanism works by turning off genes that show complete or partial similarity to the passenger strand. Researchers can overcome this issue by utilizing blunt ends and siRNA variants like asiRNA, si-siRNA, and dual-targeted siRNA alongside methods that block the 5′-end phosphorylation and deploy ss.-siRNA or aiRNA. The seed region controls the third silencing method that targets and removes genes with partial sequence matches. Solving this problem requires the use of AU-rich seeds while avoiding common seed complementation.
- Immune-stimulatory effects
Toll-like receptors (TLRs) along with non-TLR pathways function as intestinal immune sensors to detect foreign RNAs. Detection of threats prompts defense mechanisms to lead to the formation of pro-inflammatory cytokines and type I interferons. The Toll-like receptors TLR3, TLR7, TLR8, and TLR9 recognize double-stranded RNA and single-stranded RNA stimulatory motifs. The detection of specific sequences initiates signaling pathways which rely on these sequences. Researchers have discovered that specific sequence elements such as uridine and secondary structures function as immune activators along with particular sequences. The activation of Protein kinase R together with the TLR-3 pathway can happen without requiring dependency on siRNA sequence. To decrease immune stimulation during siRNA development scientists should avoid using sequences rich in guanine and polyurethane. The activation of RIG-I signaling is dependent on the presence of 5′-triphosphate in dsRNA yet replacing it with adenine lowers the immunostimulatory effects. When designing siRNA therapies the goal is to minimize immune activation but in cancer and viral treatments immunostimulatory siRNAs function as dual-action molecules by both gene silencing and triggering interferon production.
- Delivery problem
Successful siRNA delivery encounters multiple challenges at different levels even with precise design. Due to its hydrophilic nature and substantial molecular weight siRNA cannot pass through cell membranes easily. As a result of its properties siRNA becomes vulnerable to glomerular filtration followed by swift removal through the kidneys and elimination via the reticuloendothelial system. siRNA molecules degrade rapidly and this degradation rate increases when UpA sequences are present at one end. Through chemical modifications along with hydrophobic ligand incorporation and nanocarrier deployment including liposomes, lipoplexes, nucleic acid-lipid particles and various cationic polymers siRNA stability in plasma has been enhanced. Effective siRNA function requires it to surpass both extracellular and endolysosomal barriers before it arrives at the RISC. The endocytosis pathway presents challenges because the molecule's negative charge and size complicate internalization. The process of endocytosis subjects siRNA to degradation within lysosomes which requires escape strategies to enter the cytoplasm. The proton sponge effect from cationic polymers buffers endosomal pH which triggers endosome enlargement and breakage for siRNA release into the cytoplasm.
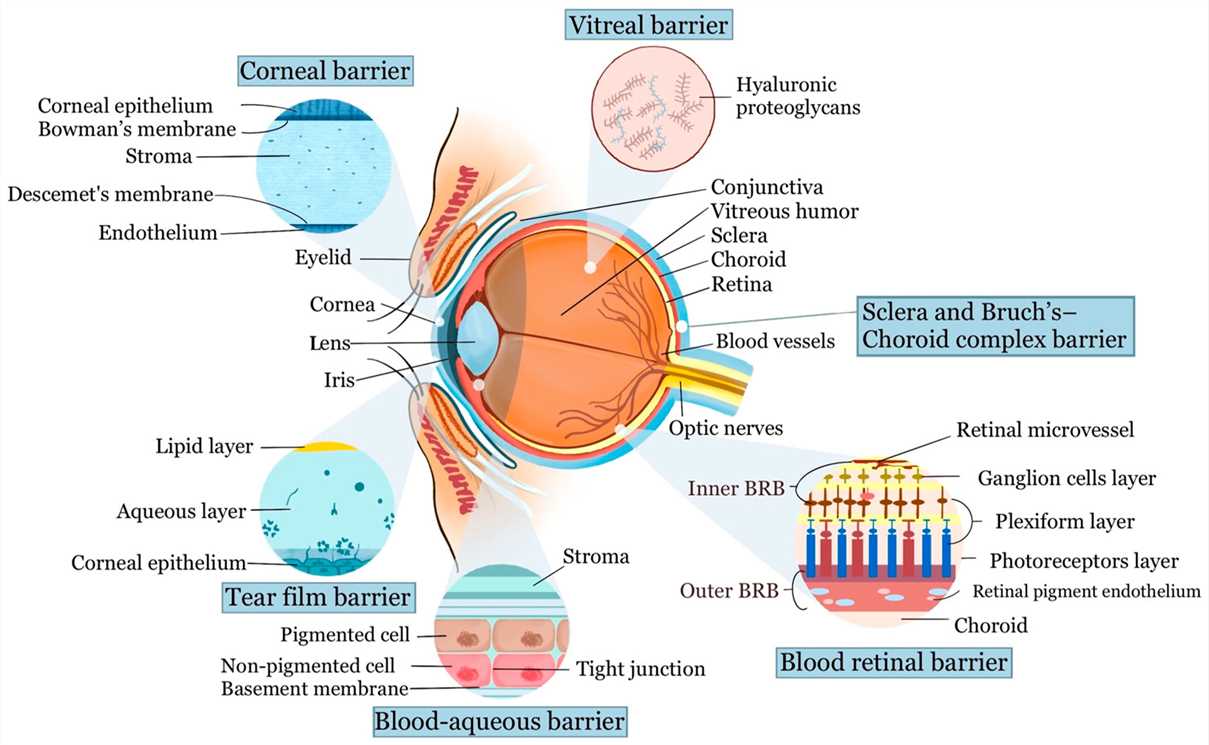 Fig. 2 Schematic diagram of the ocular anatomy and the physiological barriers to ocular drug delivery2,6.
Fig. 2 Schematic diagram of the ocular anatomy and the physiological barriers to ocular drug delivery2,6.
Strategies to enhance siRNA efficiency
- Cationic amphiphilic drugs as per siRNA delivery enhancer
Researchers conducted an investigation into an advanced method to protect siRNA from breaking down inside lysosomal membranes. The permeability qualities of cationic amphiphilic drugs enable the movement of siRNA from lysosomes to the cytosol. The effect of different CADs on primary bovine corneal epithelial cells was initially evaluated through lysosome staining combined with flow cytometry and confocal microscopy analysis of PBCECs. The research evaluated siRNA silencing effectiveness following CAD treatment to establish a link between increased siRNA transfection in PBCECs and CAD-induced lysosomal membrane permeability. The results showed that siRNA silencing efficiency increased when used together with CAD because the delivery mechanism for siRNA improved. The results suggested that administering CAD after siRNA treatment increased its gene silencing efficiency by promoting release from the lysosome.
- Chemical modification
Chemical modifications significantly influence stability and uptake of unmodified siRNA and mRNA molecules. A major advantage of these modifications lies in their ability to alter siRNA and mRNA molecules directly while maintaining their capacity to block the function of their intended targets. Researchers have studied chemical alterations of various components in siRNA and mRNA molecules including sugars and bases as well as terminals and backbones to create messenger molecules that persist longer in cells and demonstrate improved uptake. Standard practice involves making changes to the sugar component. The accessibility of siRNAs within the eye combined with their ability to target specific genes makes them highly promising for ocular therapy applications. Although siRNA therapy faces challenges it shows substantial promise for various eye diseases with many siRNA-based drugs now under clinical testing.
siRNA in preclinical models in Ophthalmic Research
- Scientists tested the delivery of chemically modified siRNA to the retinal area
The study involved alteration of twelve siRNAs through different lipid compositions and capacities. The research examined multiple molecules which all targeted Hungtintin (HTT). Researchers intravenously delivered the substance into the mice vitreous and then used molecular and histological methods to analyze its distribution throughout the retina. To determine dose-response relationships and investigate long-term safety and effectiveness researchers injected Photoreceptor (PR)-enriched siRNA into the eyes of mice and pigs. A scrambled sequence served as the non-target control group in this experiment. The siRNAs moved efficiently from the vitreous space to the posterior eye region while spreading throughout the retina. Furthermore, they were significantly enhanced in PRs. In mice and pigs HTT protein levels reduced in direct proportion to the administered dose. The PR-enriched siRNA suppression persisted for at least six months in mice retinas and lasted four months (the maximum observation period) in pig retinas. Microglia (IBa1) activation and astrocyte (GFAP) activation were not detected in retinas during long-term treatment according to histological analysis. Researchers can employ siRNA to safely and effectively target PRs for the treatment of multiple dominant mutations which cause blindness through PR degeneration.
- siRNA-VEGF for ocular neovascularization treatment
Researchers examined how siRNA packaged inside bioreducible lipidoid nanoparticles (siVEGF) affects neovascularization reduction in a rodent oxygen-induced retinopathy model after intravitreal delivery. Treatment with siVEGF lipid-like nanoparticles showed reduced expression levels of VEGF mRNA and protein and decreased both the non-perfused retinal area and retinal neovascularization extent in contrast to the untreated OIR group. Scientists created a triblock copolymer named PACD which combined hydrophilic PEG elements with siRNA binding and pH-responsive segments that formed nanosized micelles through self-assembly. Researchers injected PACD/siVEGFA polyplexes into the eyes of OIR mice to assess their effects against control groups, ranibizumab-treated mice and PACD/siNC-treated mice. Researchers found that PACD/siVEGFA polyplexes achieved the same anti-neovascularization results as ranibizumab. The PACD/siVEGFA treatment led to a substantial reduction in both mRNA and protein expression of target genes. The treatment with siVEGFA led to diminished expression of VEGFA in retinal pigment epithelium cells and in the mouse retina which resulted in decreased cell migration and retinal neovascularization. The study authors concluded that 2N12H/siVEGFA LNPs produced therapeutic results similar to those seen with ranibizumab.
References
- Ahn, Insook, Chanhee S. Kang, and Jinju Han. "Where should siRNAs go: applicable organs for siRNA drugs." Experimental & Molecular Medicine 55.7 (2023): 1283-1292. https://doi.org/10.1038/s12276-023-00998-y.
- Liu, Li-Ching, Yi-Hao Chen, and Da-Wen Lu. "Overview of recent advances in nano-based ocular drug delivery." International Journal of Molecular Sciences 24.20 (2023): 15352. https://doi.org/10.3390/ijms242015352.
- Taniguchi, Takazumi, et al. "Novel use of a chemically modified siRNA for robust and sustainable in vivo gene silencing in the retina." Scientific reports 10.1 (2020): 22343. https://doi.org/10.1038/s41598-020-79242-w.
- Ribeiro, Marcela Coelho Silva, et al. "Neuroprotective effect of siRNA entrapped in hyaluronic acid-coated lipoplexes by intravitreal administration." Pharmaceutics 13.6 (2021): 845. https://doi.org/10.3390/pharmaceutics13060845.
- Bhujel, Basanta, et al. "Current Advances in Regenerative Strategies for Dry Eye Diseases: A Comprehensive Review." Bioengineering 11.1 (2023): 39. https://doi.org/10.3390/bioengineering11010039.
- Distributed under Open Access license CC BY 4.0, without modification.
