Gene Therapy for Cystic Fibrosis
Overview of Cystic Fibrosis as a Genetic Disease
Cystic fibrosis (CF) is a multifaceted genetic disorder that has major impacts on respiratory and digestive systems and profoundly changes people's life experiences. Mutations in the CFTR gene trigger cystic fibrosis because the resulting protein directs chloride ion and water transport through cell membranes. Fluid equilibrium across multiple organs such as lungs, pancreas, liver, and intestines depends on this essential process. Mutations in the CFTR gene trigger malfunctions in its protein product which results in the creation of thick mucus that blocks airways and disrupts organ function. The lungs become infected because the thick mucus produced there creates bacterial breeding grounds which cause ongoing infections and damage to lung tissue. The malfunction in the pancreas can block digestive enzymes from being released which causes malnutrition and various digestive problems. To develop CF a person needs to receive one defective gene from each parent because it is an autosomal recessive disease that requires two copies of the defective gene. The degree of CF severity differs significantly between people because of their unique genetic mutations and additional genetic and environmental influences.
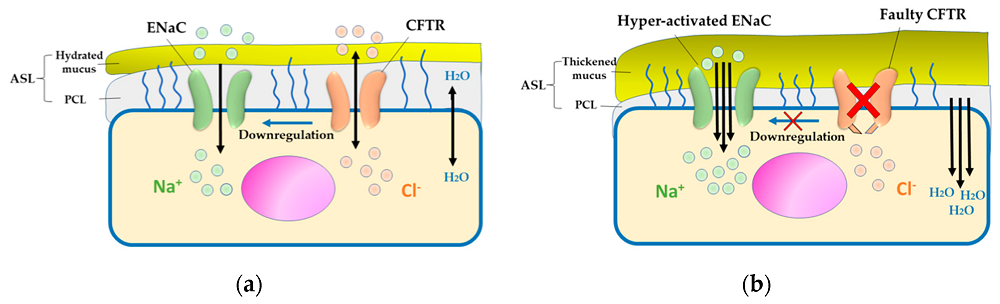 Fig. 1 Pathogenesis of cystic fibrosis (CF)1,6.
Fig. 1 Pathogenesis of cystic fibrosis (CF)1,6.
What is the Gene Therapy
The field of gene therapy continues to evolve rapidly and represents a revolutionary area of medicine which provides the means to treat numerous diseases by targeting genetic abnormalities at their root. Gene therapy functions by delivering genetic material into patient cells to address defective genes through correction or compensation. Several techniques allow this approach to function which include introducing a healthy gene to substitute for a mutated one as well as modifying genes to restore normal functionality and employing gene editing tools to precisely fix genetic mutations. Gene therapy differs from conventional treatments by targeting the fundamental source of diseases rather than just alleviating symptoms which allows for a potentially complete cure. Advancements in molecular biology along with genetic engineering and delivery technologies have enabled scientists to develop therapeutic genes with enhanced precision and efficiency, which has accelerated the progress of gene therapy development. The field of gene therapy remains new but has demonstrated its potential in successfully treating multiple genetic disorders such as inherited blindness types, severe combined immunodeficiency and specific muscular dystrophy variants.
Importance of Gene Therapy in Treating Cystic Fibrosis
Gene therapy promises to revolutionize CF treatment because it directly targets the genetic defect responsible for the condition. Standard treatments for CF work to manage symptoms and complications through antibiotics for lung infections, mucolytics that thin mucus secretions and enzyme replacement treatments for digestive problems. Existing therapies may enhance patient life experience temporarily but they do not fix the genetic root issue and often cannot make meaningful changes to the disease's long-term trajectory. Gene therapy represents a direct and potentially curative method that introduces a working CFTR gene copy to affected cells especially in the airway epithelium. Gene therapy restores CFTR protein functionality to correct ion transport defects and normalize mucus production while decreasing lung infection frequency and severity. Respiratory function would improve for patients who would also see fewer digestive complications and a better quality of life. New gene editing technologies such as CRISPR/Cas9 enable direct genomic repair of the CFTR mutation. This treatment might provide CF patients with a lifelong solution that removes their daily illness management needs.
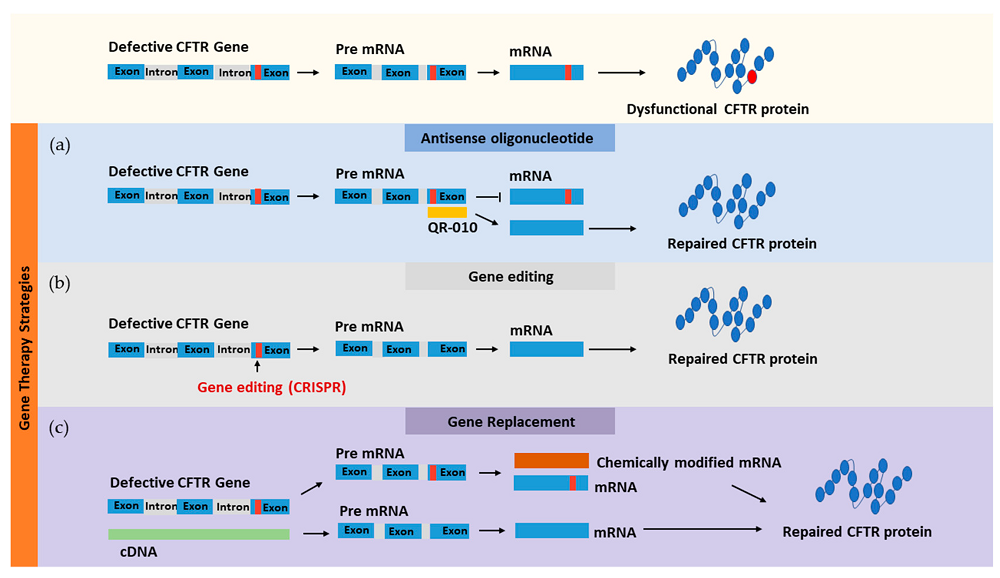 Fig. 2 Gene-based therapeutic approaches for targeting faulty CFTR using different methods: (a) antisense oligonucleotide, (b) gene editing, and (c) gene replacement1,6.
Fig. 2 Gene-based therapeutic approaches for targeting faulty CFTR using different methods: (a) antisense oligonucleotide, (b) gene editing, and (c) gene replacement1,6.
What is the Purpose of a Vector in Gene Therapy
A vector functions as an essential delivery system in gene therapy that moves therapeutic genetic material like DNA or RNA inside patient cells. Gene therapy vectors enable therapeutic gene delivery to target cells alongside cellular uptake and gene expression needed for therapeutic results. The therapeutic mechanism of gene therapy involves transporting genetic material into cells to provide treatment for genetic disorders. Cell membranes block naked DNA and RNA molecules from entering cells and accessing targeted tissues. Genetic vectors are specifically designed to navigate biological obstacles and ensure efficient delivery of therapeutic genes to target cells. Viral vectors utilize viruses' natural cell infection mechanisms to transfer genetic material while non-viral vectors use either physical or chemical approaches to enable cellular entry. Gene therapy struggles with directing the therapeutic gene exclusively to target cells while preventing unintended off-target effects. Scientists can create vectors that deliver genetic material to specific cell types or tissues. Scientists can modify viral vectors to present ligands or antibodies that attach to target cell surface receptors. Non-viral vectors such as nanoparticles may be modified with targeting molecules to ensure they reach particular tissues. Targeted delivery systems boost therapeutic effectiveness while minimizing possible side effects.
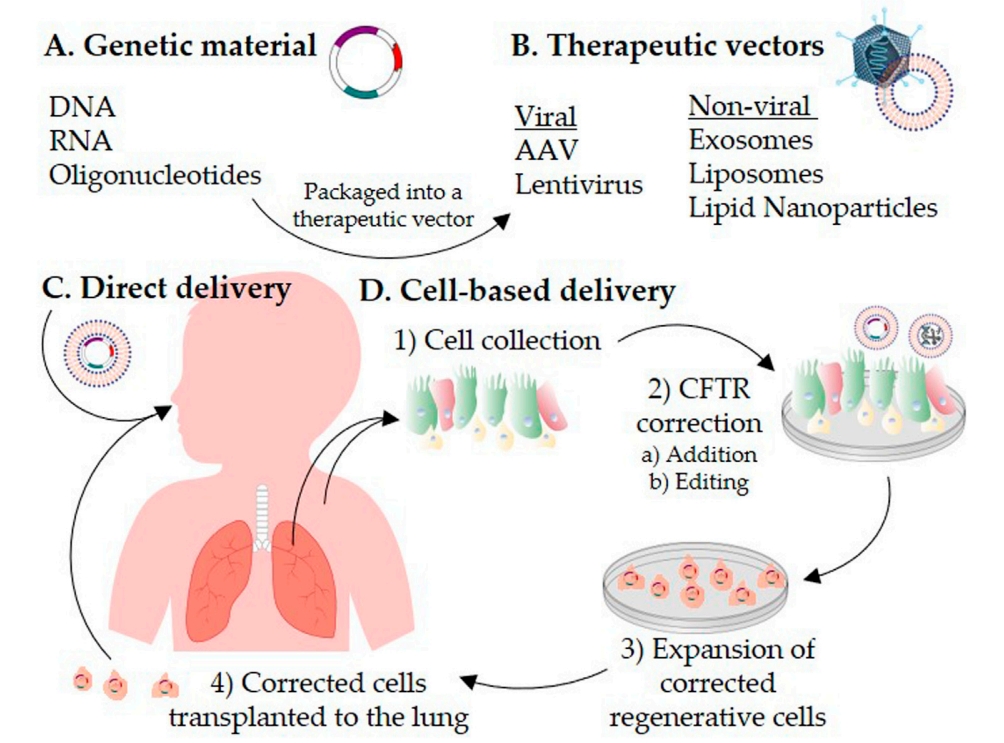 Fig. 3 Schematic representation of CFTR correction strategies for the treatment of cystic fibrosis2,6.
Fig. 3 Schematic representation of CFTR correction strategies for the treatment of cystic fibrosis2,6.
The Types of Gene Therapy Vectors
Vectors serve a critical function in gene therapy because they deliver therapeutic genetic material to targeted cells. They can be broadly categorized into two main types: viral vectors and non-viral vectors. Therapeutic requirements including target tissue specificity and gene expression duration requirements along with safety concerns determine the optimal vector type through careful evaluation of each vector's advantages and disadvantages.
- Viral Vectors
Adeno-associated virus (AAV) vectors
The origin of AAV vectors comes from a small virus which does not cause disease. The most popular viral vectors in gene therapy include these vectors because they show low immunogenicity while infecting both dividing and non-dividing cells. The low immune response triggered by AAV vectors allows for their repeated administration in therapeutic treatments.
Adenovirus vectors
The construction of adenovirus vectors originates from a medium-sized virus with a double-stranded DNA structure. These vectors excel in transduction efficiency while having the capability to infect various cell types. Adenovirus vectors demonstrate efficient delivery of genetic material into cellular environments whether cells are undergoing division or not. These vectors have the capacity to transport substantial genetic payloads up to approximately 36 kilobases.
Lentivirus vectors
Scientists create lentivirus vectors from retrovirus types like HIV which they modify to ensure safety for therapeutic applications. These vectors have the capacity to incorporate into the host's genetic material. Lentivirus vectors enable durable gene expression through the integration of therapeutic genes into the host's genomic DNA.
Retrovirus vectors
Retroviruses serve as the basis for retrovirus vectors because they belong to a group of RNA viruses which incorporate their genetic material directly into the host cell's genome. Retrovirus vectors ensure durable gene expression while efficiently targeting cells that divide quickly.
- Non-Viral Vectors
Plasmid DNA
Therapeutic genes can be transported using plasmid DNA vectors which are circular DNA molecules. Typically physical or chemical methods serve as delivery mechanisms for plasmid DNA vectors. Effective gene delivery through plasmid DNA vectors necessitates higher doses.
Nanoparticles
Nanoparticles measure between 1 and 100 nanometers and are capable of binding or encapsulating genetic material to transport it into cells. Nanoparticles generally elicit minimal immune responses.
Liposomes
Liposomes are tiny vesicles composed of lipid bilayers which serve as carriers for genetic material targeting cellular delivery. Liposomes generally elicit minimal immune responses. Liposomes exhibit biocompatibility and allow precise control over their payload release mechanism.
Current Gene Therapy Vectors for Cystic Fibrosis
- Recombinant Adenovirus(rAd) Vectors for CF
Animal models with CF tested rAd vectors as the most extensively used viral vectors. Partial correction of the Cl− transport defect in nasal epithelium for CF patients using rAd-CFTR occurred only when there was damage to the nasal epithelium during delivery despite respiratory tract infections being the primary sign of adenovirus infection. The coxsackievirus and adenovirus receptor (CAR) which enables adenoviruses type-2 and -5 to attach and infect cells resides on the basolateral membrane of human airway epithelium. The rAd vector demonstrates two major disadvantages when used for CFTR expression in post-mitotic airway cells because its effects are short-lived and it triggers both cellular and humoral immune responses that lead to destruction of treated cells while blocking repeated treatments. Preexisting Pseudomonas infection in a host increases anti-adenovirus immune responses because this infection is a characteristic of CF.
- Helper-dependent Adenovirus Vectors for CF
To address the limitations of rAd in human gene therapy systems scientists developed helper-dependent adenovirus vector (HD-Ad). The HD-Ad removes all viral-encoded genes which eliminates T-cell responses to hidden viral protein expression resulting in reduced inflammation and extended gene expression within the airways. Research demonstrated that HD-Ad vectors could successfully express CFTR in CF knockout mice lungs and achieve efficient transduction of large experimental animals such as rabbits and ferrets through aerosol delivery. HD-Ad vectors with their spacious 37 kb capacity make it possible to transport a gene editing endonuclease system and donor DNA for homologous recombination together in one vector. The technology allows for gene cassette movement from rAd genome to chromosome through piggyBac transposase-mediated integration or programmable nuclease-mediated targeted insertion which solves transient expression problems.
- Recombinant Adeno-associated Virus Vectors for CF
The first decade of AAV-based CF gene therapy saw the use of only rAAV2 as the available serotype vector. The airways do not naturally harbor AAV2 yet its application in lung gene transfer proved viable based on its broad tropism which enabled preclinical studies to show productive transduction of rabbit and rhesus macaque lungs using rAAV2. Subsequent research involving rAAV2 transduction biology using polarized human airway epithelium in cell culture models at an airway-liquid interface revealed rAAV2's poor transduction rates of human epithelium through the apical membrane. The rAAV2 vector performed apical transduction in HAE-ALI at 100 times lower efficiency compared to polarized cultures from rhesus macaque primary airway epithelial cells. Research showed that rAAV2 transgene expression was 200 times less when infecting apically than basolaterally in HAE-ALI yet detected significant quantities of rAAV2 genomes after apical entry. Researchers found no association between vector entry and transgene expression which indicated that the primary cause of inefficiency was not vector binding and endocytosis but instead the inability to achieve productive transduction.
- Lentiviral Vectors for CF
Lentiviral vectors originating from immunodeficiency viruses target both active and inactive cells and ensure continuous transgene expression from the integrated viral DNA throughout the lifespan of the recipient cells. The risk of insertional mutagenesis remains a safety concern despite the absence of evidence from clinical trials with lentiviral vectors. The lung airways do not naturally host lentiviruses so scientists must pseudotype lentiviral vectors with suitable envelope proteins for airway transduction. The UK CFGTC has launched a phase I/IIa lentiviral vector gene therapy trial for CF using rSIV.F/HN-hCEF-CFTR because non-viral strategies face challenges with low transfection efficiency and temporary expression. The rSIV.F/HN-hCEF-CFTR vector contains the CFTR expression cassette from pGM169 and uses F and HN proteins from the Sendai virus (SeV) for pseudotyping. The lentiviral method rSIV.F/HN-hCEF-CFTR outperformed GL67A formulated pGM169 by several log orders in its ability to transduce airway epithelial cells.
- Preclinical Analysis of Gene Therapy in Animal Models of CF
Researchers created numerous CF mouse models immediately following the discovery of CFTR. The CF mice that carry CFTR mutations fail to develop spontaneous lung infections or inflammation rendering them inadequate as animal models for testing CF lung disease gene therapies. Large-animal CF models have provided a resolution for this ongoing issue. The lung disease phenotypes found in CF patients are replicated by CFTR knock-out (KO) ferrets and pigs. Researchers showed proof-of-concept using lentivirus-, rAd- and rAAV-mediated CFTR lung gene transfer in CFTR-KO pigs to demonstrate gene therapy possibilities in CF lung conditions. CF develops as a progressive disease but lung disease phenotypes appear later and are not present at newborn or young ages in both ferret and pig models. In ferrets it takes over a year to develop an infected CF lung. Severe gastrointestinal defects present before and after birth in both pig and ferret CFTR-KO models lead to significant rearing challenges because newborn survival rates remain low. AAV vectors demonstrate potential for CF gene therapy because they produce minimal immune response and sustain long-term gene expression. Research with ferrets and pigs has shown that AAV vectors can successfully deliver the CFTR gene to cells that line the airway passages.
Challenges of Gene Therapy for Cystic Fibrosis
- Efficient Gene Delivery
Gene therapy for cystic fibrosis struggles to deliver therapeutic genes to target cells like the airway epithelial cells effectively. In CF patients, the dense mucus layer in their lungs obstructs vector access to target cells by forming an effective physical barrier. The airway epithelium exhibits rapid cellular turnover which creates a dynamic environment that challenges sustained gene expression. The latest research using ferrets and pigs demonstrates that despite the advanced capabilities of AAV vectors, uniform and efficient transduction of airway epithelial cells continues to be difficult to achieve.
- Immune Responses
Viral vector-induced immune responses pose a major obstacle in the advancement of gene therapy techniques. The repeated use of viral vectors such as adenovirus and lentivirus generates immune responses that lower treatment efficacy and create potential side effects. The need for sustained gene expression creates significant challenges for CF treatment using current gene delivery systems. Strong immune responses during preclinical studies with adenovirus vectors resulted in the elimination of the vector and diminished gene expression levels.
- Disease Progression and Model Limitations
Animal models show different disease progression rates than humans which makes it difficult to apply preclinical findings to clinical settings. Animal models cannot completely represent all human CF characteristics because they fail to reproduce the intricate interactions between genetic mutations and environmental influences. Ferret and pig models have provided insights into pulmonary disease yet they might not accurately represent the persistent nature of human CF or the enduring impacts of gene therapy.
Future Directions in Gene Therapy for Cystic Fibrosis
- Optimizing Delivery Systems
Research going forward needs to concentrate on creating more effective delivery methods that precisely target their intended destinations. Researchers must improve viral vectors to enable them to breach the mucus barrier and effectively transduce airway epithelial cells. Researchers are evaluating non-viral delivery methods like nanoparticles and liposomes because they can transport bigger genetic materials while causing fewer immune responses. Preclinical research has shown that nanoparticles with specialized targeting ligands can successfully deliver therapeutic genes directly to airway epithelial cells.
- Genome Editing Technologies
Genome editing tools like CRISPR/Cas9 provide a possible curative solution by making direct corrections to CFTR gene mutations within the genome. The approach functions to reinstate normal CFTR gene activity to establish a lasting treatment solution. Animal model research supports the potential of CRISPR/Cas9 to repair CFTR mutations but faces obstacles in delivering the editing system both efficiently and safely.
- Combination Therapies
Gene therapy in combination with established CFTR modulators could improve therapeutic effectiveness. CFTR modulators demonstrate potential benefits in CFTR function enhancement for certain patients while their combination with gene therapy creates opportunities for more effective treatment methods. Current clinical research focuses on combining CFTR modulators with gene therapy to improve treatment results for patients with cystic fibrosis.
References
- Almughem, F.A.; Aldossary, A.M.; Tawfik, E.A.; Alomary, M.N.; Alharbi, W.S.; Alshahrani, M.Y.; Alshehri, A.A. Cystic Fibrosis: Overview of the Current Development Trends and Innovative Therapeutic Strategies. Pharmaceutics. 2020, 12, 616. https://doi.org/10.3390/pharmaceutics12070616.
- Allan, K.M.; Farrow, N.; Donnelley, M.; Jaffe, A.; Waters, S.A. Treatment of Cystic Fibrosis: From Gene- to Cell-Based Therapies. Front. Pharmacol. 2021, 12:639475. https://doi.org/10.3389/fphar.2021.639475.
- Lomunova, M.A.; Gershovich, P.M. Gene therapy for Cystic Fibrosis: recent advances and future prospects. Acta Naturae (англоязычная версия), 2023, 15(2): 20-31. https://doi.org/10.32607/actanaturae.11708.
- Sui, H.; Xu, X.; Su, Y.; Gong, Z.; Yao, M.; Liu, X.; Zhang, T.; Jiang, Z.; Bai, T.; Wang, J.; Zhang, J.; Xu, C.; Luo, M. Gene therapy for cystic fibrosis: Challenges and prospects. Front. Pharmacol. 2022, 13:1015926. https://doi.org/10.3389/fphar.2022.1015926.
- Lee, JA.; Cho, A.; Huang, E.N. Gene therapy for cystic fibrosis: new tools for precision medicine. J Transl Med. 2021, 19, 452. https://doi.org/10.1186/s12967-021-03099-4.
- Distributed under Open Access license CC BY 4.0, without modification.
