Revolutionizing Medicine: How In Vivo Cell Therapy Works
The New Frontier of Cellular Medicine
In modern medicine in vivo cell therapy marks a pivotal shift towards a novel era of disease treatment. This innovative therapy stands out distinctly from traditional methods foregoing the ex vivo process of extracting manipulating and re-introducing cells. Instead, it targets direct modification of cells within the body's natural environment offering a promising and groundbreaking alternative for addressing various medical conditions.
In vivo cell therapy is a cutting-edge medical technique that treats illnesses by directly altering cells inside the patient's body, doing away with the necessity to remove and manipulate cells outside the body (ex vivo). This technique introduces therapeutic medicines precisely into target cells by utilizing cutting-edge delivery technologies like viral vectors and nanoparticles. In vivo cell therapy is a less invasive and maybe more effective option for treating genetic disorders, malignancies, and neurological diseases because it operates in the body's natural environment. Its capacity to precisely fix flaws at the cellular level is what gives it its transformational promise.
The emergence of in vivo cell therapy is closely linked to the progress of sophisticated cell therapy technologies which form the foundation of this transformative approach. These technologies facilitate the accurate delivery of therapeutic agents to the intended cells. Various delivery platforms have been engineered to cater to this need. Notably viral vectors particularly adeno-associated viruses (AAV) and lentivirus have emerged as potent delivery carriers for genetic material. AAV is renowned for its minimal immunogenicity and capacity to achieve prolonged gene expression rendering it a favorite for gene-based therapies. Lentivirus conversely boasts the capability to integrate genetic material into the host cell's genome thereby proving useful for applications demanding stable gene transfer. This development underscores the pivotal role of advanced technologies in driving the progress of in vivo cell therapy1.
Lipid-based nanoparticles have become a promising delivery system for cell therapy. Among other medicinal payloads, these nanoparticles can incorporate DNA and RNA. Their design can be tailored to enhance stability boost cellular uptake and even target specific cell types. Furthermore, hybrid biological-synthetic systems are under investigation which amalgamate the benefits of biological components such as cell-targeting ligands with synthetic materials. These systems combine the advantages of biological components, like cell - targeting ligands, with synthetic materials, offering the potential for highly customized and efficient delivery.
How In Vivo Cell Therapy Works
Bypassing the complications of ex vivo techniques, in vivo cell therapy delivers therapeutic chemicals straight to target cells inside the body. The procedure starts with the creation of specialized delivery systems that contain genetic material or other therapeutic payloads, including lipid-based nanoparticles or viral vectors (like lentiviruses or adeno-associated viruses). These vectors are designed to target certain tissues utilizing tissue-specific promoters and to avoid immune detection, frequently by employing covert coatings like polyethylene glycol (PEG).
After being administered, the vectors find their way to the targeted cells and discharge their cargo. This may entail replacing a damaged gene with a functional one in cases of hereditary diseases. In cancer treatment, immune-modulating drugs or modified viruses can boost the body's immune response or target tumors specifically. Cutting-edge methods like CRISPR-Cas9 allow for precise gene editing inside the body, fixing mutations at their root cause.
To ensure safety and effectiveness, the entire process depends on state-of-the-art production conducted in accordance with stringent Good production Practice (GMP) guidelines. Ongoing developments in AI-optimized vector design and intelligent feedback mechanisms hold the potential to substantially enhance and broaden the uses of in vivo cell treatment, transforming contemporary medicine, notwithstanding obstacles such as high prices and scalability.
Breakthrough Delivery Technologies
In order to ensure that therapeutic drugs reach their targets precisely and efficiently, sophisticated delivery systems are essential to the success of in vivo cell treatment.
Reaching tailored delivery is one of the main areas of innovation. Several strategies have been developed. Tissue - specific promoters play a crucial role in this regard. One pivotal component is tissue-specific promoters which are DNA sequences capable of controlling gene expression. By incorporating these promoters into vectors gene expression can be confined to the intended organ. For example, certain liver diseases can be targeted using a promoter that activates exclusively in liver cells. This approach enhances treatment accuracy and effectiveness by minimizing unintended effects and restricting therapeutic gene expression to the liver.
Stealth coatings are another important innovation. The immune system continuously monitors for foreign bodies. When delivery vectors are introduced, they may be recognized as foreign and attacked by the immune system. Vectors can be coated with stealth materials, such as polyethylene glycol (PEG), to evade immune detection. This shielding extends their circulation time in the body, improving the likelihood of successful delivery to target cells.
Dual - vector systems have also been developed to address the challenge of delivering larger genetic payloads. Some therapeutic applications require the delivery of multiple genes or large pieces of genetic material. Dual - vector systems can be designed in such a way that they work in tandem to deliver these larger or more complex genetic cargos. For example, one vector can carry a gene - editing tool, while the other can carry the template DNA for precise gene editing.
Manufacturing Considerations
The production of in vivo cell therapies is a complex process that demands specialized equipment and strict adherence to Good Manufacturing Practice (GMP) standards. Figure 1, shows a closed - system bioreactor used for GMP production.
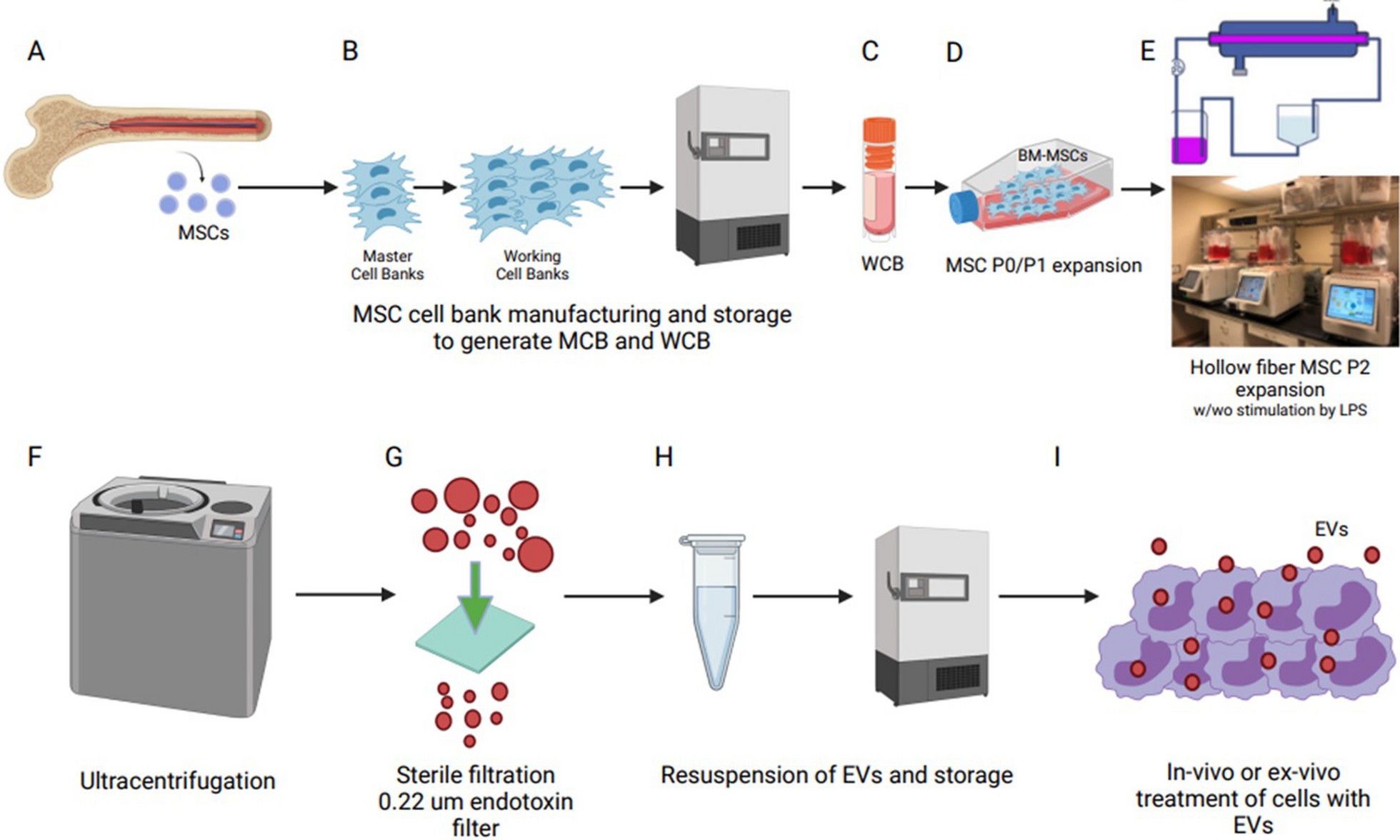 Fig 1. Proposed GMP manufacturing platform for MSC-EV production. The picture outlines a GMP manufacturing platform for MSC - EV production, starting with isolating mesenchymal stromal cells from bone marrow, followed by cell banking, expansion, EV isolation, concentration, filtration, and final testing for regulatory - compliant clinical use. In essence, it presents a streamlined process to ensure the quality and regulatory adherence of MSC - EVs for in vivo or ex vivo clinical applications3.
Fig 1. Proposed GMP manufacturing platform for MSC-EV production. The picture outlines a GMP manufacturing platform for MSC - EV production, starting with isolating mesenchymal stromal cells from bone marrow, followed by cell banking, expansion, EV isolation, concentration, filtration, and final testing for regulatory - compliant clinical use. In essence, it presents a streamlined process to ensure the quality and regulatory adherence of MSC - EVs for in vivo or ex vivo clinical applications3.
Single - use bioreactor bags are a key component in this setup. Designed for single-time use they mitigate the risk of cross-contamination between batches. Moreover, they ease the cleaning and sterilization processes safeguarding the product's quality. Automated monitoring sensors integrated into the system continuously oversee essential parameters such as temperature pH and oxygen levels offering real-time data. These sensors provide real - time data, allowing operators to make immediate adjustments to ensure optimal cell growth conditions.
Integrated purification modules are indispensable in the GMP manufacturing system. Post-culturing and therapeutic agent production purification is vital to eliminate impurities like host cell proteins nucleic acids and endotoxins. The purification modules in the bioreactor system efficiently accomplish these tasks guaranteeing the final product aligns with the rigorous quality standards for clinical use. Nevertheless the manufacturing of in vivo cell therapies faces significant challenges including high production costs scalability issues and ensuring batch-to-batch consistency which the industry is actively addressing2.
Clinical Impact
In vivo cell therapy offers promising applications in medicine, particularly for genetic disorders once deemed untreatable. By introducing a functional copy of the defective gene directly into affected cells, this method could address conditions caused by single-gene mutations.
This therapy also shows significant promise for neurodegenerative diseases like Parkinson's and Alzheimer's. Delivering neurotrophic factors or genes that enhance neuronal survival and function to the brain could potentially halt or even reverse disease progression.
As an emerging alternative to conventional cancer treatments—such as radiation, chemotherapy, and surgery—in vivo cell therapy employs engineered oncolytic viruses to selectively attack tumors while sparing healthy tissue, thereby modifying the tumor microenvironment.
Furthermore immune-modulating agents can be delivered to the tumor site bolstering the body's natural immune response against cancer cells.
Future Directions
The field of in vivo cell therapy is constantly evolving, and several emerging areas of research are on the horizon. AI - optimized vector design is one such area. Machine learning algorithms can analyze vast amounts of data related to vector structure, function, and cell - targeting capabilities. Using this data AI predicts the optimal vector design for a given therapeutic application thereby reducing the time and cost associated with traditional vector development techniques.
Research into CRISPR delivery in vivo is also fascinating. CRISPR-Cas9 technology has revolutionized gene editing, and it in vivo delivery could open up new therapy options for hereditary diseases. By carefully changing genes inside the body, it may be possible to correct mutations at their source and provide a more potent treatment approach.
Smart therapies with feedback mechanisms are also being explored. These therapies can sense the body's physiological state and adjust therapeutic agent delivery accordingly. To ensure more focused and effective therapy, a biomarker-sensing vector, for example, may identify disease-related biomarker levels and release the chemical only when a certain threshold is achieved.
Conclusion
The development of in vivo cell therapy marks a major milestone in medicine, enabling direct cellular modification to combat diseases with unprecedented precision. Despite its transformative potential, this innovative approach still faces significant challenges. While distribution methods need to be further refined to ensure optimal efficacy and safety, the production process needs to be optimized for cost reduction and better scalability. Nevertheless, the swift progress in research and development within this realm is undeniably promising. Through sustained investment and ongoing innovation in vivo cell therapy has the capacity to revolutionize disease treatment ultimately enhancing the quality of life for countless patients. This transformative potential underscores the significance of continued advancements in this field.
References
- Krug, A., Ane, A. S., Martinello, C., Fusil, F., Michels, A., Buchholz, C. J., Ricci, J. E., & Verhoeyen, E. (2024). In vivo CAR T cell therapy against angioimmunoblastic T cell lymphoma. Journal of Experimental & Clinical Cancer Research, 43(1), 262. https://doi.org/10.1186/s13046-024-03179-5
- Helfer, B. M., Ponomarev, V., Patrick, P. S., Blower, P. J., Feitel, A., Fruhwirth, G. O., Jackman, S., Mouriès, L. P., Park, M. V. D. Z., Srinivas, M., Stuckey, D. J., Thu, M. S., Van den Hoorn, T., Herberts, C. A., & Shingleton, W. D. (2021). Options for imaging cellular therapeutics in vivo: a multi - stakeholder perspective. Cytotherapy, 23(9), 757 - 773. https://doi.org/10.1016/j.jcyt.2021.02.005
- Kink, J.A., Bellio, M.A., Forsberg, M.H. et al. Large-scale bioreactor production of extracellular vesicles from mesenchymal stromal cells for treatment of acute radiation syndrome. Stem Cell Res Ther 15, 72 (2024). https://doi.org/10.1186/s13287-024-03688-2
