ARC-521
Hepatitis B virus (HBV) infection is a major global cause of liver cirrhosis and hepatocellular carcinoma and poses a constant threat to the health of people around the world. Currently available drugs can inhibit viral replication in most patients, but only about 10% can clear viral antigens. In recent years, the emerging RNA interference is a gene-level treatment method. It uses small fragments of RNA molecules to specifically silence genes. This silence can reduce or even block the replication of specific proteins, thereby achieving the purpose of treating diseases.
ARC-521 is an RNAi therapeutic agent for chronic hepatitis B infection, which can be seen as a supplement to ARC-520, used to interfere with the upstream of the hepatitis B virus reverse transcription process. Although ARC-521 performed well in a series of early efficacy tests, and showed high safety, due to the potential safety hazards of the R & D company's RNAi platform, the drug trials related to ARC-520 and ARC-521 are temporarily suspended.
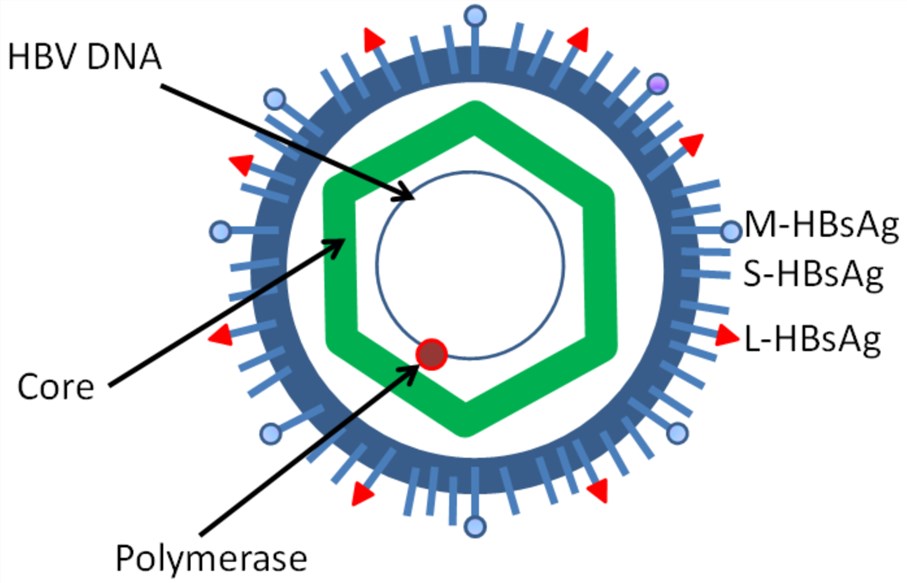 Figure 1. The structure of hepatitis B virus.1
Figure 1. The structure of hepatitis B virus.1
Hepatitis B Virus Infection
According to the latest estimates, 240 million people worldwide are carriers of HBV surface antigen (HBsAg). The form of HBV infection may range from acute hepatitis B (AHB) to persistent active or inactive infection, which may lead to cirrhosis and hepatocellular carcinoma (HCC). The process of HBV infection may be affected by many factors, including co-infection with other hepatitis viruses or human immunodeficiency virus (HIV), hepatotoxic factors including alcohol intake, and HBV genotypes. The stage of HBV infection can be determined based on the presence of two major viral antigens (HBsAg and HBeAg) in the patient's blood, HBV DNA levels and alanine aminotransferase (ALT) activity.
HBV infection can be divided into five stages to reflect the relationship between the host immune system and the virus. Chronic HBV infection begins with a high HBeAg-positive replication phase, characterized by high HBV DNA levels and normal or slightly elevated ALT activity, followed by a variable but still HBeAg-positive immunoreactivity phase, in which HBV DNA is low and ALT activity is elevated. The third stage is the inactive HBV vector (low replication) phase, characterized by loss of HBeAg, with anti-HBe seroconversion, low HBV DNA levels and normal ALT. Most patients at this stage have detectable mutations in the HBV pronuclear/core promoter gene, leading to aggravation of the intermittent disease and associated remission period, which is typical of the fourth stage of HBeAg negative chronic hepatitis. Finally, some patients have entered the stage of HBsAg-negative occult HBV infection, which is characterized by low or undetectable HBV DNA, with a reduced risk of cirrhosis and liver failure, but due to the presence of free forms of HBV, cccDNA, and additionally the risk of HBV reactivation in case of immunosuppression.
ARC-521 is a Supplement to ARC-520
Since the current treatment options cannot be used universally to solve the health problems caused by chronic HBV infection, some companies have started the discovery of gene therapy and developed some small RNA molecules that can silence the expression of HBV virus genes.
Among them, ARC-520 is the best product in clinical and animal experiments, with a knockout rate of 99% for HBsAg and a knockout rate of 70% for HBeAg. ARC-521 is a siRNA product developed specifically for HBeAg. It can access the normal cell RNAi mechanism of the human body and specifically cut off HBV RNA transcription, thereby reducing the level of HBV protein and making RNA templates for viral DNA. As a supplement to ARC-520, ARC-521 can act on the transcription process of HBV cccDNA and integrated viral DNA, and is suitable for chronic hepatitis B patients with low HBV cccDNA levels. By evaluating whether ARC-521 can significantly reduce circulating and non-circulating viral proteins and RNA, it is expected to effectively reconstruct the host's immune response and eventually achieve HBsAg serum clearance to achieve functional cure for hepatitis B.
If you want to know more about this product, you can contact us for more details.
Reference
- From Wikipedia: Dr Graham Beards, CC BY-SA 3.0, https://en.m.wikipedia.org/wiki/File:HBV.png.
