TKM-130803
Background
The pathophysiology of Ebola virus disease (EVD) in humans is characterized by shock, coagulopathy, vascular leakage, and multi-organ injury, with severity closely correlated with Ebola virus (EBOV) RNA levels in the blood. Though there are no treatment modalities or approved vaccine available for preventing EBOV infections, a few post-exposure approaches have demonstrated convincing efficacy against EBOV in a nonhuman primates (NHPs) model. These approaches include anti-EBOV monoclonal antibody (mAb) administration alone (such as ZMapp) or with adenovirus-vectored interferon-α, and EBOV-targeting small interfering RNAs (siRNAs) encapsulated in lipid nanoparticles (LNPs) to potentiate cellular delivery. The bipartite structure of the formulation, comprising siRNA and LNP, allows for adjustments to the siRNA component to capitalize on emerging strain sequence data while maintaining the delivery functionality of the LNP component. Once viral sequence data is available, clinical-grade drug products can be produced within 8 weeks.
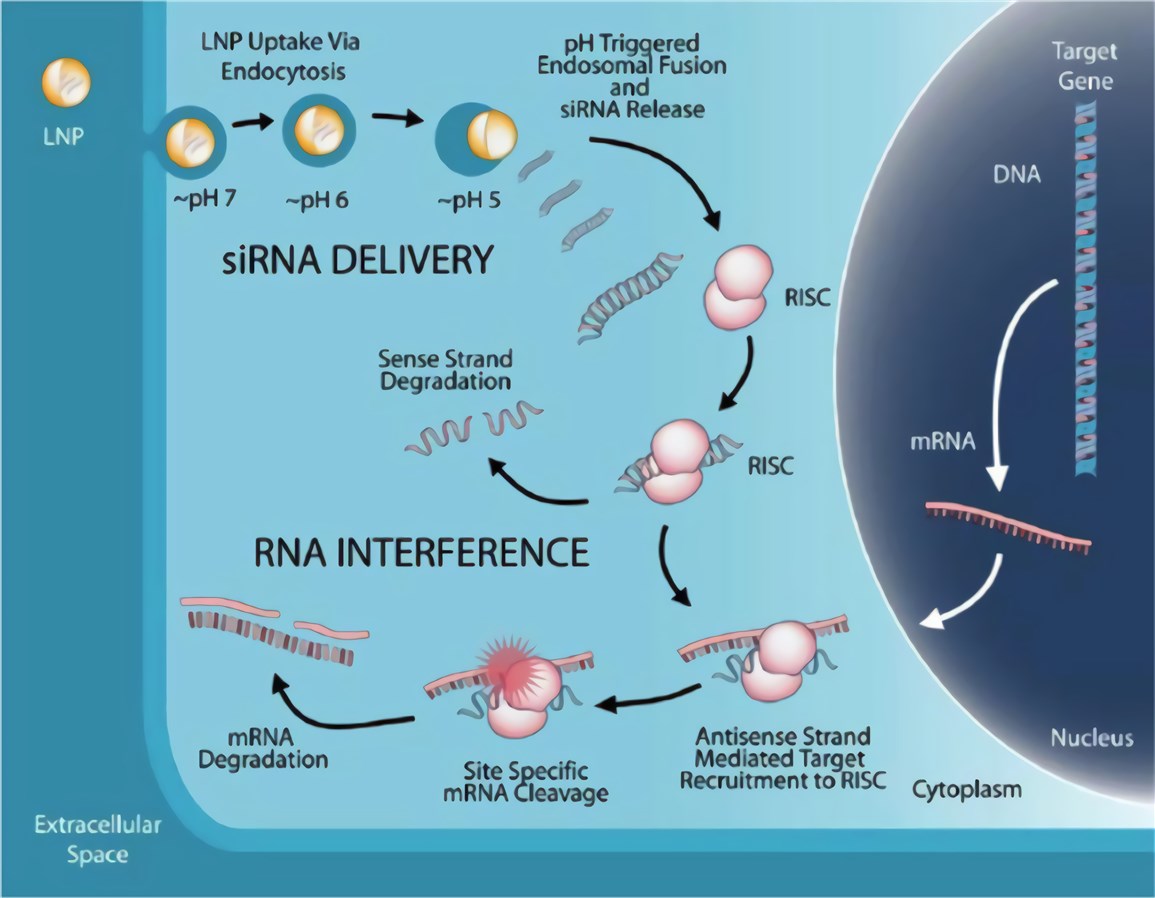 Figure 1. Mechanism of LNP-mediated RNA interference (RNAi) therapeutic. (Scott, 2020)
Figure 1. Mechanism of LNP-mediated RNA interference (RNAi) therapeutic. (Scott, 2020)
TKM-130803
One of the lead experimental therapies prioritized for evaluation by WHO was TKM-100802, an LNP formulation of siRNAs directed against the gene products encoding two viral proteins. One is L polymerase (Lpol), involved in transcription and replication of Zaire ebolavirus and the other is viral protein-35 (VP35), involved in suppression of the host immune response. As for TKM-130803, it is a new formulation of TKM-100802 in which the siRNA component has been adapted by two nucleotide substitutions in the VP35 siRNA and a single nucleotide substitution in the L-polymerase siRNA to ensure specificity to the West African Makona variant of Zaire ebolavirus causing the 2014-2015 West African outbreak.
Phase II Clinical Trial
- Methods
- Conclusion
In the single-arm phase II trial, adults with laboratory-confirmed EVD received 0.3 mg/kg of TKM-130803 by intravenous infusion once daily for up to 7 days. On days when trial enrolment capacity was reached, patients were enrolled in a concurrent observational cohort.
Administration of TKM-130803 at a dose of 0.3 mg/kg/d by intravenous infusion to adult patients with severe EVD was not shown to improve survival when compared to historic controls. The trial cannot identify whether the drug is both ineffective and harmful since the futility rule terminated the trial because the survival in those receiving TKM-130803 was no better than historic survival rates.
Further work is needed to assess whether the lack of observed effects is generalizable to other patient subgroups in other treatment settings. Additionally, the potential influence of drug formulation and dose requires further investigation. Please contact us for more information about TKM-130803.
Reference
- Scott, J. T.; et al. (2020). Pharmacokinetics of TKM-130803 in Sierra Leonean patients with Ebola virus disease: plasma concentrations exceed target levels, with drug accumulation in the most severe patients. EBioMedicine. 102601. Distributed under Open Access license CC BY 4.0, without modification.
