Unlocking the Blueprint-Mastering the Art of siRNA Design for Precision Gene Silencing
Definition of siRNA
RNA interference (RNAi) is a natural biological process where small interfering RNAs (siRNA) duplexes act to induce a powerful inhibition of genes. Naturally occurring siRNA duplexes are generated when the enzyme Dicer cleaves long dsRNA into shorter pieces. siRNA fragments (21-23 nucleotides long) that are formed by this process associate with a complex containing RNase and form the RNA-induced silencing complex (RISC). The duplex dissociates and releases the sense strand. The antisense strand remains associated with RISC, and guides the activated complex to its target complementary mRNA. RISC continues to cleave and degrade the mRNA in a subsequent step. Minimal quantities of siRNA are required to reduce the amount of mRNA and consequently reduce expression of the target protein in a particular disease phenotype. RNAi is rapidly being adopted by the researcher/medical community as a method for targeted gene expression knockdown. Gene silencing is becoming a popular approach in functional genomics. Improved kits and protocols for siRNA reagents are facilitating quick and convenient RNAi experiments.
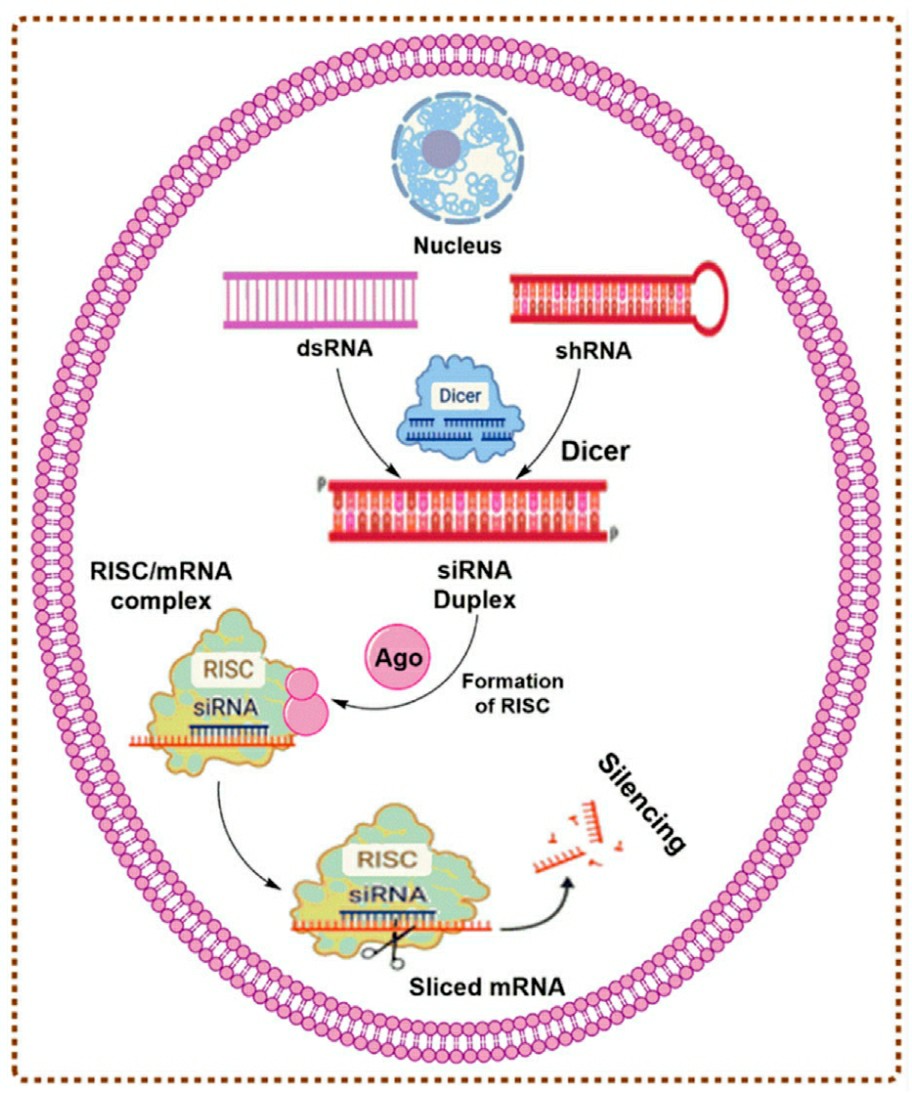 Fig. 1 The mechanism of gene silencing by siRNAs1,4.
Fig. 1 The mechanism of gene silencing by siRNAs1,4.
Design Principles of siRNA
- Target site
SNP positions are excluded as the preferred siRNA design site because the siRNA would not be equally efficient in different cell lines with different single nucleotide polymorphisms (SNPs). SNPs should not be considered as the site for siRNA designing. Also, it is not advisable to consider intronic part or 5'UTR or 3'UTR as siRNA design sites. UTRs and areas adjacent to the start codon are more likely to bind with regulatory proteins, which could hinder RISC complex and its silencing effect. Target sites should be 50-100 nucleotides downstream of the start codon in the open reading frame of the target gene, which are the most effective. SiRNAs that target regions close to the start codon are more effective than those targeting far from the start codon. It is also worth checking for the motifs 5'-AA(N19)TT, 5'-AA(N21) or 5'-NA(N21) in the target sequence as GC content of these regions are <50%; they also facilitate the RISC complex function. Recent research shows that the availability of the target site is another important factor to determine the functionality of siRNA and the slight change in the partial base pairing of the target site would affect the siRNA function.
- Length of siRNA and Specificity checking
The optimal length of siRNAs is a topic of debate. Although some people have tried 19 nucleotide siRNAs and obtained excellent results, other people have used longer siRNAs (21–29 nucleotides). Shorter siRNAs might cause unspecific binding, but 19–25 nucleotide siRNAs showed the same level of silencing as long as 21–29 nucleotides. The smaller the siRNAs are, the better it is to use them in mammalian cells, because longer siRNAs may induce mammalian immune response. After designing siRNAs by different methods, both sense and antisense strands should be checked by blast using the reference sequence database (Refseq-RNA database) of the desired organism to decrease the possibility of silencing of the unintended genes. Since their alignment may not result from the coincidence, the blast's results with small E-values should not be ignored. If the query covers less than 78% of other genes and ⩽15 nucleotides out of 19 of the corresponding siRNA match with the other gene, we think that it is tolerable. However, it is worth mentioning that the off-target effect is always unpredictable for siRNAs.
- Nucleotide content of siRNA
One of the simple and primary parameters to consider for the efficient siRNA is GC content. This is because low GC content causes non-specific and weak binding, and high GC content may hinder unwinding of the siRNA duplex by helicase and RISC complex. However, various reports indicated different acceptable ranges of GC content. For instance, some researchers reported that GC content should be in the range of 31.6–57.9%. Others reported that the majority of functional siRNAs (up to 95%) have GC content ranging from 36 to 52%. Additionally, researchers reported a more accurate study and indicated that the GC percentages in the antisense strand between the second to the seventh and the eighth to the eighteenth nucleotides should be 19% and 52%, respectively. Moreover, functional siRNAs have an unstable region (less GC content than other regions) between the ninth to the fourteenth nucleotides called energy valley that is another significant factor for selecting siRNAs. This internal instability increases the functionality of the RISC complex by giving the best conformation during mRNA cleavage.
siRNA delivery systems: strategies for endosomal escape
The virus-based delivery systems have shown good transfection properties. However, a large number of problems have been associated with these delivery systems. As a result, there has been an increasing interest in the use of non-viral nanoparticle-based delivery systems for effective siRNA delivery. The difficulties in siRNA delivery technology can be caused by extreme temperatures, which can inhibit the activities of nucleic acid-based drugs inhibiting specific targeted effects. Encapsulation of siRNAs within nano-vesicles can aid in protecting the siRNAs from destruction by nucleases and the immune response of the host enabling successful delivery of siRNAs. Delivery of siRNAs to their target cells can be enhanced by nano-ligand bound vesicles.
- Lipid-based carriers
Lipid-based siRNA delivery systems face several in vivo barriers for transportation of their siRNA cargo to target cells. One barrier is the maintenance of lipoplex stability in the bloodstream. A variety of liposome modifications have been developed to protect positively charged fusogenic liposomes from interactions with serum proteins and macrophages to maintain the fusogenic character necessary for efficient endosomal escape and intracellular trafficking of the siRNAs. The ratio of lipid and siRNA can be optimized to generate a slightly positive zeta potential for the lipoplex to facilitate its interaction with the negatively charged cell membrane and the subsequent cellular internalization. The toxicogenomics of cationic lipids including the initiation of immune responses and changes in the expression of non-target genes are known. Intravenous injection of siRNA loaded in liposomes of cationic and fusogenic lipids yielded continued siRNA existence and decreased the mRNA and protein levels of the two targeted genes, CD31 and Tie2, in the vasculature endothelium in different organs. Intraperitoneal injection of cationic liposomal anti-TNF-α siRNA to mice inhibited lipopolysaccharide-induced TNF-α gene expression and prevented the development of sepsis encouraged by a subsequent lipopolysaccharide (LPS) injection.
- pH-sensitive polyplexes
Polyplexes must destabilize intracellular vesicle membranes for permeating them, but must not disintegrate other cell surface, mitochondrial, or nuclear membranes. Moreover, knowing of biological acidification in endo-lysosomes or some extracellular tumor regions has motivated scientists to include pH-sensing constituents, such as acid-labile bonds, to change the polyplex characteristics. Several pH-sensitive, endosomal buffering polymers have been created as nonviral delivery systems for the improvement of siRNA delivery. A multifunctional polymeric siRNA carrier, [1,4,7-triazanonylimino-bis[N-(oleicyl-cysteinyl-histinyl-1-aminoethyl)propionamide] (THCO)] that contains a proton sponge domain, a hydrophobic domain and cysteine residues that allow polymerization has been developed. The different protonable amino groups with various pKas permitted complexing of siRNA molecules, allowing a high buffering capacity. These nanoparticles had pH-sensitive membrane disruption capability under acidic conditions. The hydrophobic part of the particle improved the stability of the carrier by forming a tight structure with the nucleic acids inside it. Furthermore, the disulfide bonds' reduction by cytosolic glutathione led to the dissociation of nanoparticles and siRNA release into the cytoplasm.
Applications of siRNA in Diseases
- Cancer Therapy
Lung cancer is one of the most common cancers and a leading cause of cancer death in men and the second leading cause of cancer death in women. Besides surgery and other adjuvant therapies, the novel siRNA therapeutics is being developed against the molecular targeted pathway. Another factor is drug resistance in the treatment of cancer. In a study in 2017, researchers developed a EGFR targeted chitosan-nanoparticle carrying chemotherapeutic drug Cisplatin along with siRNA targeting the Mad2 gene which is responsible for the cellular mitosis. The IC50 of drug alone was 140.73 µM on drug-resistant human lung adenocarcinoma cells (A549-DDP) whereas IC50 of cisplatin reduced to 0.094 ± 0.0023 µM and 0.057 ± 0.031 µM with siMad2 at 1nM and 5nM, respectively. Lethality of ovarian cancer arises from several mechanisms and AXL protein is one of the contributors to ovarian cancer metastasis. Researchers in 2019 developed a novel carrier p5RHH, derived from the modification of natural peptide melittin by chemical methods. The system contained siRNA targeting AXL, which was able to inhibit the invasion and migration of ovarian cancer cells in vitro. In the xenograft mouse model of ovarian cancer, p5RHH-siAXL decreased metastasis without any toxicity.
- Hypertension
Hypertension is a common condition worldwide affecting 1.28 billion adults globally and 46% of those are not aware they have the condition. Zilebesiran is a siRNA which suppresses the hepatic production of angiotensinogen (AGT) via asialoglycoprotein receptor. Angiotensin II production is then decreased, subsequently decreasing blood pressure. Zilebesiran possesses favourable pharmacokinetic properties. One dose produces prolonged activity. Zilebesiran has shown marked reductions in blood pressure in clinical trials with a continued effect for up to 24 weeks post-injection. Combination therapy with angiotensin receptor blockers has shown additional benefit in hypertension treatment. Zilebesiran also has a favorable safety profile, most adverse events are manageable injection site reactions. Zilebesiran is a novel agent in the treatment of hypertension with a high degree of blood pressure reduction with manageable adverse events and convenient dosing characteristics.
- Lung injury
Recently, scientists delivered the same naked siRNA and liposomal encapsulated siRNA into the trachea and the vein, respectively, for the downregulation of PD-L1 (Programmed death ligand-1) which was highly upregulated in pulmonary endothelial cells (ECs) and epithelial cells (EpiCs) after hemorrhagic shock followed by cecal ligation and puncture septic challenge (Hem-CLP)-induced ALI in mice model. The treatment with naked siRNA inhibited EpiCs but not ECs. However, PD-L1 siRNA treatment by liposome inhibited ECs but not EpiCs. As a result, i.v. administration of liposomes loaded with the same siRNA inhibited the development of Hem-CLP induced ALI. These effects were not found when naked siRNA was administered intratracheally.
- Infectious Diseases
The siRNA drugs are also developed for other purposes. For example, the anti-viral siRNA drug RG6346 knockdowns the viral hepatitis B surface antigen, which is a protein necessary for the hepatitis V virus life cycle, in liver hepatocytes. Four-monthly doses of RG6346, given in a phase I study, resulted in a large and sustained decrease in hepatitis B surface antigen levels, persisting up to 1 year after the last dose. A phase II study of RG6346 as monotherapy or in combination with other investigational anti-hepatitis B virus drugs/approved long-term hepatitis B virus treatment was started in March 2021. Most recently, the first siRNA drug for COVID-19 was tested in clinical trials. In vitro, the most effective for inhibition of viral replication siRNA targeting the RNA-dependent RNA polymerase of the severe acute respiratory syndrome coronavirus 2 was identified. This siR-7 was formulated with a peptide dendrimer (KK-46). In a Syrian hamster model for severe acute respiratory syndrome coronavirus 2 infection, a large decrease in virus titers and lung inflammation was observed in the animals when exposed to inhalation of siR-7-EM/KK-46.
- Intracerebral hemorrhage (ICH)
A separate group of researchers indirectly targeted the cell death pathway in a mouse ICH model by targeting vascular adhesion protein 1 (VAP-1) via siRNA. VAP-1 promotes leukocyte migration and infiltration across the blood brain barrier (BBB) into the brain parenchyma during an inflammatory state. Silencing VAP-1 by siRNA 24 h prior to ICH resulted in decreased leukocyte migration, lessened cerebral edema, and improved neurobehavioral deficits. These results suggest that VAP-1 siRNA might be a target for inflammation after ICH. Additionally, TWEAK (TNF-like weak inducer of apoptosis) binding to fibroblast growth factor-inducible 14 (Fn14) activates the inflammatory element, JAK/STAT. Activating the JAK/STAT pathway is the pathway that mediates neuroinflammation in neurodegenerative disease. In a rat ICH model, overexpression of long non-coding RNA small nucleolar RNA host gene 3 (Snhg3), which regulates cell proliferation and dysfunction of brain microvascular endothelial cells, increased expression of TWEAK and Fn14. Silencing Snhg3 by siRNA (injected 24 h after ICH and every 2 days until day 7) reduced brain edema and cell apoptosis, enhanced BBB integrity, and improved neurobehavioral outcome by silencing the TWEAK/Fn14/STAT3 pathway.
References
- Goyal, Rajat, et al. "Insights on prospects of nano-siRNA based approaches in treatment of Cancer." Frontiers in pharmacology 13 (2022): 985670. https://doi.org/10.3389/fphar.2022.985670.
- Lück, Stefanie, et al. "siRNA-Finder (si-Fi) software for RNAi-target design and off-target prediction." Frontiers in Plant Science 10 (2019): 1023. https://doi.org/10.3389/fpls.2019.01023.
- Tai, Wanyi. "Current aspects of siRNA bioconjugate for in vitro and in vivo delivery." Molecules 24.12 (2019): 2211. https://doi.org/10.3390/molecules24122211.
- Distributed under Open Access license CC BY 4.0, without modification.
