While the clinical successes of CAR T-cell therapies are profound, their widespread application is currently limited by a spectrum of severe adverse effects (SAEs). These toxicities are primarily driven by the uncontrollable activation of CAR T-cells, manifesting as excessive immune responses due to unregulated action time and off-target effects stemming from improper spatial localization. Among these, neurotoxicities represent a particularly critical and complex challenge.
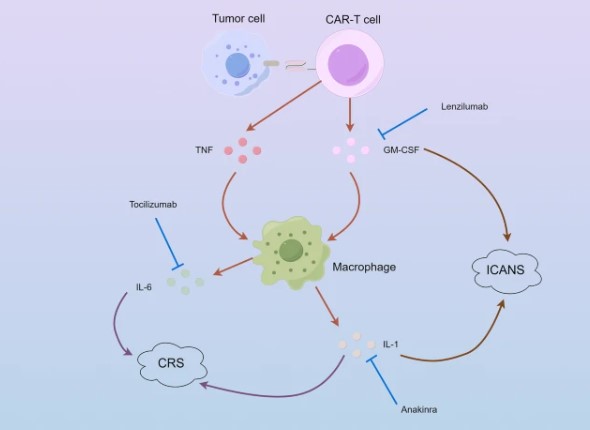 Fig.1 CRS and ICANS mechanisms and potential therapeutic drugs.1
Fig.1 CRS and ICANS mechanisms and potential therapeutic drugs.1
At Creative Biolabs, we specifically focus on mitigating Tumor Inflammation-Associated Neurotoxicity (TIAN), a distinct toxicity syndrome observed in patients treated with cellular therapies and other immunotherapies for tumors located within the central nervous system (CNS). Unlike the more generalized Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) seen with systemic CAR-T therapies, TIAN is uniquely characterized by inflammation associated with CNS tumor activity. TIAN is directly relevant to the unique challenges of treating CNS malignancies with targeted immunotherapies, necessitating specialized management strategies.
Creative Biolabs offers an integrated suite of services under our TIAN Management Solutions, designed to support the development of safer and more effective CAR T-cell therapies from discovery to clinical translation, with a specific emphasis on managing CNS-related toxicities. Our service content is meticulously crafted to address the multifaceted challenges of CAR-T toxicity, particularly mitigating TIAN:
Creative Biolabs offers advanced platforms for the identification of predictive and prognostic biomarkers associated with TIAN. Utilizing cutting-edge multi-omics technologies, including proteomics, genomics, metabolomics, and multiplex cytokine profiling, we screen patient samples and preclinical CNS tumor models to identify early molecular signatures indicative of impending neurotoxicity. This enables proactive patient management, allows for personalized risk assessment, and guides timely intervention before severe neurological symptoms or imaging changes consistent with TIAN manifest.
Beyond mere identification, Creative Biolabs provides robust services for the rigorous validation of putative TIAN biomarkers. Through comprehensive analytical and clinical validation studies, employing diverse patient cohorts with CNS tumors and advanced statistical methodologies, we confirm the reliability, sensitivity, and specificity of these biomarkers. This critical step ensures that identified markers are truly predictive and can be translated into clinically actionable diagnostic and prognostic tools, strengthening the foundation for precision TIAN management.
Our dedicated service provides in-depth mechanistic investigations into CAR-T cell-induced neurodamage using sophisticated preclinical models. We employ highly relevant in vitro systems, such as human brain organoids and neuro-inflammatory co-cultures, alongside advanced in vivo humanized mouse models designed to mimic CAR T-cell infiltration and activity in the CNS. Through comprehensive histopathological analysis, advanced imaging techniques, and functional behavioral assessments, we elucidate the specific cellular and molecular pathways underlying TIAN, identifying potential targets for intervention and validating novel therapeutic strategies to prevent or reverse neurodamage.
Creative Biolabs offers specialized services to study the intricate interplay between CAR T-cells, the broader immune system, and neurotoxicity in controlled experimental settings pertinent to CNS tumors. Utilizing immunodeficient animal models, which can be reconstituted with human immune components or bear human CNS tumor xenografts, we meticulously analyze local and systemic immune responses, cytokine storm dynamics, and T-cell activation profiles in relation to the manifestation of neurotoxic symptoms specific to the CNS. This service is instrumental in dissecting the immune drivers of TIAN, assessing the impact of different CAR constructs on immune cell interactions within the CNS, and evaluating immunomodulatory strategies for toxicity control.
Q1: What is Tumor Inflammation-Associated Neurotoxicity (TIAN)?
A1: TIAN is a specific neurotoxicity syndrome distinct from CRS and ICANS, observed in patients receiving immunotherapies, particularly cellular therapies, for central nervous system (CNS) tumors. It is characterized by inflammation within the CNS, which can lead to cerebral edema and neurological symptoms, sometimes mimicking tumor progression.
Q2: What role does synthetic biology play in Creative Biolabs' solutions for TIAN?
A2: Synthetic biology is fundamental to Creative Biolabs' approach to TIAN management. We use genetic engineering techniques to construct artificial regulatory circuits and control modules within CAR T-cells. These modules enable the precise temporal and spatial control necessary to enhance the safety and specificity of the therapy, directly contributing to the mitigation of neurotoxic events in the CNS.
Q3: Are next-generation CAR T-cells developed by Creative Biolabs safer for CNS tumors, especially concerning TIAN?
A3: Yes. Creative Biolabs' next-generation CAR T-cells are specifically engineered to be safer for CNS tumors, with a strong focus on minimizing TIAN. Strategies like logic-gated designs (e.g., dual antigen-sensing) require multiple signals for activation, significantly increasing specificity and reducing off-target toxicities in the complex tumor microenvironment, thereby lowering the risk of neuroinflammation and TIAN.
To learn more about Creative Biolabs' Tumor Inflammation-Associated Neurotoxicity (TIAN) Management Solutions and how we can partner to advance your therapeutic programs for CNS tumors, please contact us today. Partner with Creative Biolabs to redefine the future of cancer immunotherapy – a future where efficacy is matched by unparalleled safety and control, particularly in addressing complex neurotoxicities like TIAN in CNS malignancies.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION