On-target/Off-tumor Toxicologic Effect Analysis Service
Online Inquiry
Background Service Highlights FAQs Contact Us
The Critical Imperative: Navigating CAR-T Cell Therapy Toxicities
Chimeric Antigen Receptor (CAR) T-cell therapy has revolutionized oncology, offering unprecedented clinical efficacy in treating refractory hematologic malignancies. This groundbreaking immunotherapy harnesses the patient's own T cells, genetically engineered to target and eliminate cancer cells expressing specific antigens. Despite its transformative potential, the widespread application of CAR-T cell therapy is significantly challenged by the emergence of treatment-related toxicities. These adverse events, which range in severity from mild to life-threatening, necessitate a profound understanding and robust mitigation strategies to ensure patient safety and expand therapeutic reach.
A primary concern in CAR-T development is the phenomenon of on-target/off-tumor toxicity. This occurs when CAR-T cells, designed to recognize an antigen highly expressed on tumor cells, inadvertently attack healthy tissues that express the same or a structurally similar antigen at lower levels. Such unintended recognition can lead to severe organ damage, underscoring the critical need for meticulous preclinical and clinical evaluation of CAR-T constructs. The complexity of CAR-T cell mechanisms, involving intricate signaling pathways and potent effector functions, demands sophisticated analytical approaches to precisely characterize their safety profiles. At Creative Biolabs, with over two decades of expertise in biological research and development, we recognize that comprehensive toxicologic effect analysis is not merely a regulatory hurdle but a scientific imperative for the successful translation of next-generation CAR-T therapies.
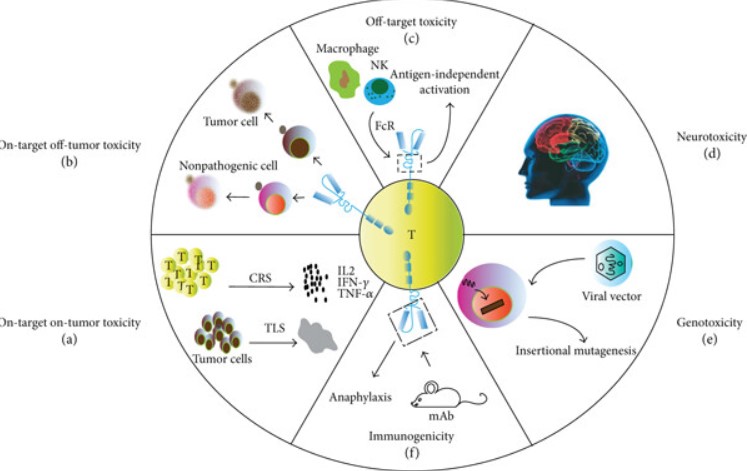 Fig.1 CART-related toxicities.1
Fig.1 CART-related toxicities.1
Our On-target/Off-tumor Toxicologic Effect Analysis Service for Next-Gen CAR-T Toxicity Management Solutions
The development of safer and more effective CAR-T cell therapies hinges on the ability to accurately predict and characterize potential on-target/off-tumor toxicities early in the drug discovery pipeline. Creative Biolabs' specialized On-target/Off-tumor Toxicologic Effect Analysis Service is meticulously designed to address this critical need, providing comprehensive solutions for next-generation CAR-T toxicity management. Our service goes beyond standard safety assessments, delving into the intricate molecular and cellular mechanisms underlying unintended CAR-T activity.
We understand that the identification of target antigens for CAR-T therapy is a double-edged sword: while high tumor expression is desirable for efficacy, even low-level expression on normal tissues can trigger devastating side effects. Our approach at Creative Biolabs integrates advanced in vitro and in vivo models with state-of-the-art analytical platforms to provide a holistic view of CAR-T cell specificity and potential off-tumor reactivity. This proactive identification of risk factors allows developers to refine CAR constructs, optimize dosing strategies, and implement effective risk mitigation measures, ultimately accelerating the path to safer and more efficacious therapies.
Comprehensive Service Contents
Creative Biolabs' On-target/Off-tumor Toxicologic Effect Analysis Service offers a multi-faceted approach to characterize the safety landscape of your CAR-T candidates. Our service content includes, but is not limited to:
Antigen Expression Profiling on Healthy Tissues
Utilizing highly sensitive techniques such as quantitative PCR, Western blot, immunohistochemistry (IHC), and flow cytometry, we meticulously quantify target antigen expression across a panel of normal human tissues. This provides a crucial baseline for assessing the risk of on-target/off-tumor toxicity.
In Vitro Specificity and Cytotoxicity Assays
We perform co-culture assays using CAR-T cells and target cells expressing varying levels of the target antigen (both tumor and healthy primary cells). Endpoints include:
-
Cytokine Release Quantification
Measuring pro-inflammatory cytokines (e.g., IL-6, TNF-α, IFN-γ) via ELISA or multiplex bead arrays, indicative of CAR-T activation.
-
CAR-T Cell Proliferation and Activation Markers
Analyzing CAR-T cell expansion and upregulation of activation markers (e.g., CD69, CD25) in response to antigen exposure.
In Vivo Toxicity Models
For lead candidates, Creative Biolabs offers robust in vivo models utilizing immunocompetent or humanized mouse models. These studies evaluate:
Histopathological analysis of major organs for signs of CAR-T mediated damage.
-
Pharmacokinetic/Pharmacodynamic (PK/PD) Analysis
Tracking CAR-T cell persistence, expansion, and cytokine profiles in vivo.
-
Biomarker Discovery and Validation
We leverage proteomics and transcriptomics to identify novel biomarkers associated with on-target/off-tumor toxicity, enabling earlier detection and monitoring of adverse events.
Featured Platform: Advanced Technologies for Precision Toxicology
At Creative Biolabs, our commitment to precision and accuracy is underpinned by our state-of-the-art analytical platforms. Our featured platform for on-target/off-tumor toxicologic effect analysis integrates cutting-edge technologies to deliver unparalleled insights:
High-Throughput Flow Cytometry
Equipped with multi-parameter flow cytometers, we enable rapid and comprehensive phenotyping of CAR-T cells, assessment of target cell antigen expression, and quantification of cell death and activation markers. This allows for efficient screening of multiple CAR constructs and target cell lines.
Multiplex Cytokine and Chemokine Profiling
Our advanced Multiplex bead-based immunoassay and MSD platforms allow for simultaneous quantification of dozens of cytokines and chemokines from small sample volumes, providing a detailed snapshot of the inflammatory response triggered by CAR-T cell activity.
Advanced Imaging Systems
High-content imaging platforms facilitate detailed morphological analysis of cellular interactions and tissue damage, offering visual evidence of on-target/off-tumor effects.
FAQs
Q1: What types of CAR-T constructs can Creative Biolabs analyze?
A1: Creative Biolabs' service is adaptable to various CAR-T constructs, including different scFv domains, co-stimulatory molecules, and signaling domains. We can also assess novel CAR designs and gene-edited T cells.
Q2: How does Creative Biolabs differentiate between on-target/off-tumor and off-target toxicities?
A2: Our comprehensive approach combines antigen expression profiling, in vitro specificity assays with varying antigen levels, and in vivo models to meticulously distinguish between on-target/off-tumor effects (due to antigen expression on healthy tissues) and true off-target effects (unrelated to the intended antigen).
Contact Us
At Creative Biolabs, we are dedicated to advancing the field of CAR-T cell therapy by ensuring the highest standards of safety and efficacy. Our On-target/Off-tumor Toxicologic Effect Analysis Service is your strategic partner in developing next-generation CAR-T solutions. To learn more about how Creative Biolabs can support your CAR-T development program and to discuss your specific project needs, please contact us today.
Reference
-
Sun, Shangjun et al. "Immunotherapy with CAR-Modified T Cells: Toxicities and Overcoming Strategies." Journal of immunology research vol. 2018 2386187. 17 Apr. 2018, doi:10.1155/2018/2386187. Distributed under Open Access License CC BY 4.0, without modification.






 Fig.1 CART-related toxicities.1
Fig.1 CART-related toxicities.1




