CAR-T therapy has emerged as a groundbreaking approach in the immunotherapy landscape. Given the complexity and specificity of CAR-T therapy, the production of high-quality materials such as in vitro transcribed RNA (IVT RNA) is crucial. IVT RNA plays an essential role in delivering genetic information into T cells, facilitating the expression of CARs. At Creative Biolabs, we are committed to providing top-tier IVT RNA manufacturing services specifically optimized for CAR-T applications. Our advanced platform ensures precision and efficiency, supporting researchers and clinicians in the development and deployment of cutting-edge CAR-T therapies.
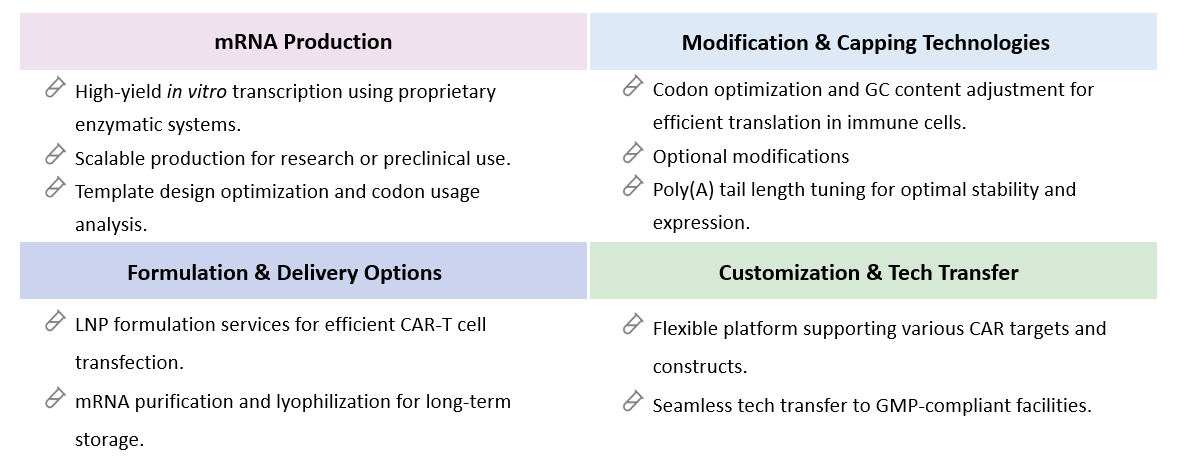
At Creative Biolabs, our IVT RNA platform is meticulously designed to meet the diverse needs of researchers and clinicians in developing of CAR-T therapy. This platform integrates state-of-the-art technologies and stringent quality control measures to ensure the production of high-fidelity mRNA transcripts, which are crucial for effective CAR-T cell engineering. Utilizing a comprehensive IVT RNA platform, our experts proudly provide high-quality mRNA tailored for research purposes, ensuring that the initial stages of CAR-T and other advanced therapies are founded on reliable and effective mRNA constructs. By employing our RG mRNA manufacturing platform, researchers can confidently advance their CAR-T studies, armed with the assurance that their mRNA constructs are of the highest quality.
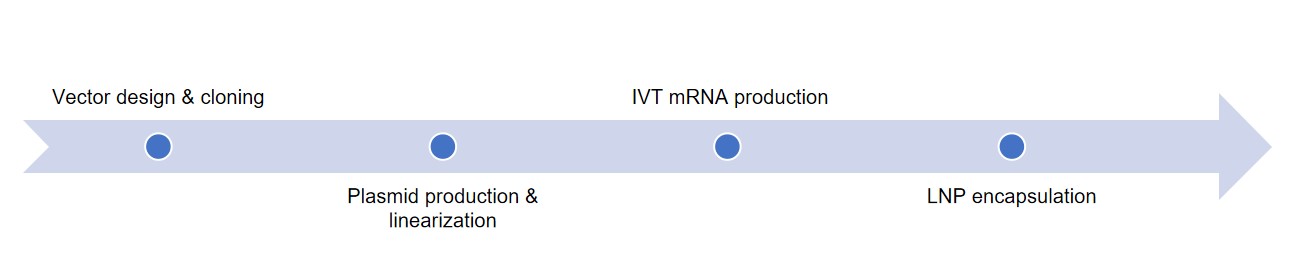
At Creative Biolabs, we provide an integrated mRNA manufacturing platform specifically optimized for CAR-T and other cell-based immunotherapies. Our advanced capabilities cover every stage of mRNA production - from DNA template design to purified, translation-ready mRNA - ensuring high yield, reproducibility, and regulatory compliance.
To guarantee the integrity, reproducibility, and safety of our research-grade CAR-T mRNA products, Creative Biolabs performs a full panel of in-process controls and release tests. Each mRNA batch undergoes strict analytical validation to ensure compliance with the key quality attributes required for advanced cell therapy applications.
| Category | Purpose | Test Item |
|---|---|---|
| Identity | Confirms correct mRNA construct and sequence fidelity | mRNA length verification (Agarose gel electrophoresis / Capillary electrophoresis) |
| Sequence confirmation (NGS or Sanger sequencing) | ||
| Integrity | Ensures full-length mRNA without degradation | RNA integrity number analysis |
| Capillary electrophoresis profile | ||
| Purity | Confirms removal of impurities and template DNA | HPLC / UV-spectrophotometry (A260 / A280 ratio) |
| dsRNA contamination assay (dot-blot or ELISA) | ||
| Residual DNA / protein quantification (qPCR / BCA assay) | ||
| Potency / Functionality | Verifies that the mRNA produces a functional protein in target cells | In-vitro translation assay |
| Cell transfection efficacy (flow cytometry or fluorescence readout) | ||
| Safety | Ensures product is non-pyrogenic and microbe-free | Endotoxin level (LAL test, ≤ 0.25 EU/µg) |
| Bioburden test (USP <61>) | ||
| Residual Impurities | Confirms removal of process-related materials | Enzyme residuals (T7 polymerase ELISA) |
| NTP residual assay |
Why should I choose the mRNA platform over established lentiviral vectors for my early-stage CAR-T research?
mRNA offers rapid, cost-effective production and, critically, transient expression, which provides an intrinsic safety switch for managing early toxicity. Furthermore, our non-viral method eliminates the risk of insertional mutagenesis associated with integrating vectors, streamlining your regulatory de-risking process.
How scalable is Research Grade mRNA production, and what is the path to GMP manufacturing?
Our RG platform is inherently scalable, moving easily from milligram-scale for early research to multi-gram quantities required for clinical development. The core synthesis and purification processes utilized in our RG service are designed to be compatible with our GMP manufacturing protocols, ensuring a smooth, low-risk technology transfer when you are ready to advance to clinical trials.
What measures does Creative Biolabs take to address the concern of mRNA immunogenicity?
Immunogenicity is primarily driven by double-stranded RNA (dsRNA) contaminants. We employ advanced multi-step purification techniques, including HPLC and TFF, to ensure the final mRNA product achieves exceptional purity, significantly minimizing dsRNA levels and ensuring a low innate immune response in your sensitive cell cultures.
Using Creative Biolabs' Research Grade (RG) CAR-T mRNA Manufacturing Service in our preclinical safety assays has significantly reduced off-target cytokine release. The removal of dsRNA contaminants was clearly superior to our in-house IVT methods, resulting in cleaner T cell profiles and more reliable in vivo data. Dr. La FD.
The transient nature of the CAR-T mRNA facilitated toxicity modeling in our solid tumor program. We could precisely control the duration of CAR expression, which was invaluable for establishing a crucial safety window and allowed us to mitigate potential CRS risks before moving to stable transduction methods. Jas Rz.
At Creative Biolabs, we are committed to advancing the field of CAR-T therapy through our specialized IVT RNA and LNP manufacturing services. Our robust RG mRNA manufacturing platform combines scalability, high purity, customization, advanced quality control, and efficient delivery systems to meet the diverse needs of CAR-T researchers. By partnering with us, you gain access to cutting-edge technologies and expert support, empowering you to drive innovation and make significant strides in the fight against cancer.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION