Are you encountering challenges related to APC targeting and activation in improving immunotherapy effectiveness? Our APC antigen presenting cell (APC) targeting enhancement service addresses these critical barriers through proprietary checkpoint regulation technology, enabling accelerated development of precision immunotherapies.
Immunotherapy has transformed modern cancer treatment, and its clinical success depends on effectively activating immune components - particularly antigen-presenting cells (APCs). While gene therapy shows promise, existing approaches struggle to achieve precise APC targeting and sustained activation. This challenge intensifies as tumor microenvironments actively impair APC functionality through immunosuppressive mechanisms, compromising adaptive immunity. Developing innovative delivery systems capable of overcoming these biological barriers represents an urgent priority to advance next-generation immunotherapies.
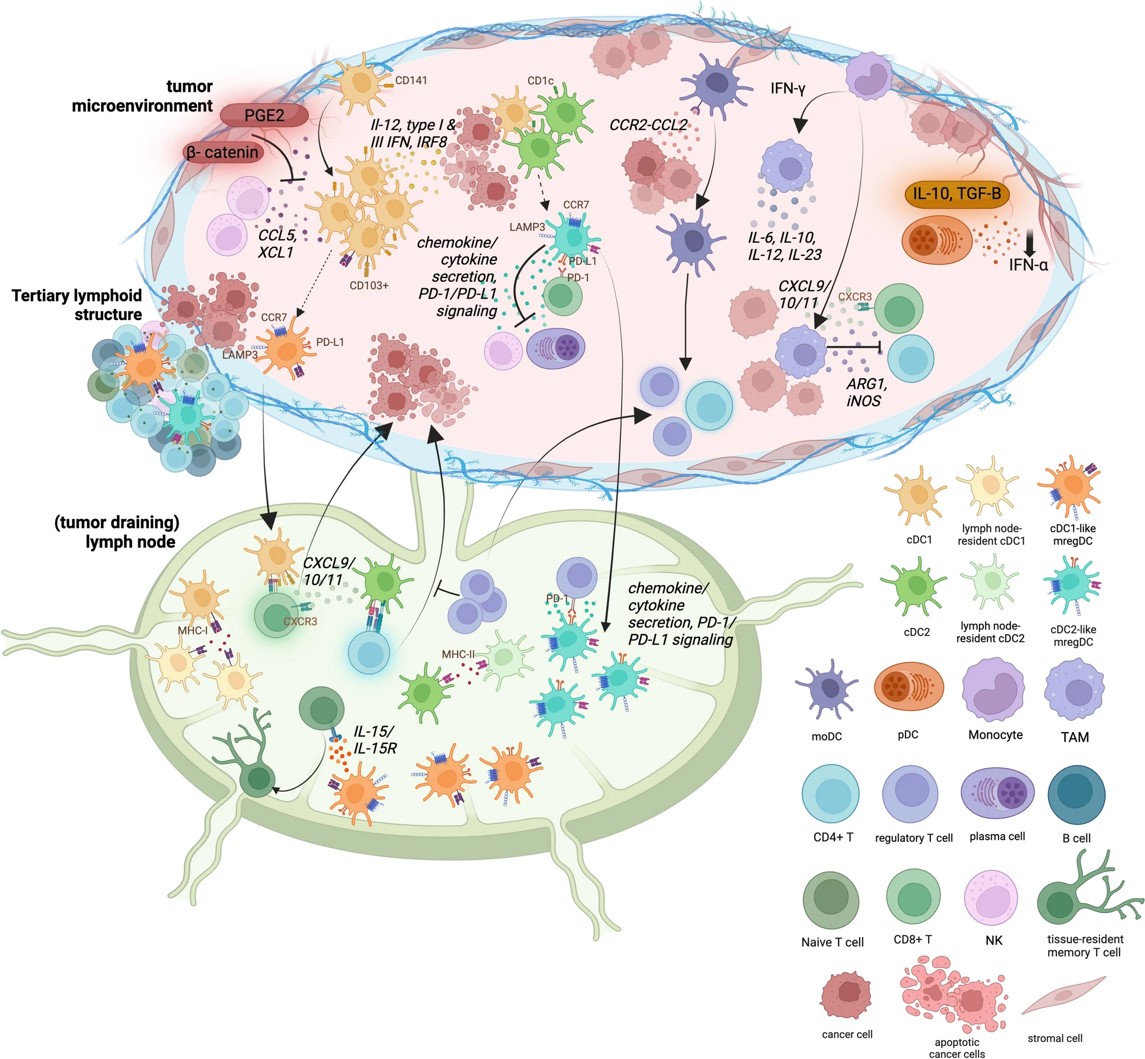 Fig.1 Tumor microenvironment immune cell cross-talk.1
Fig.1 Tumor microenvironment immune cell cross-talk.1
Creative Biolabs' active checkpoint-controlled APC targeting enhancement service advances immunotherapy innovation by enabling selective engagement of dendritic cells, B-cells, and related antigen-presenting populations. The methodology employs configurable assembly architectures to achieve disease-specific immunogen transport, promoting Th1-polarizing cytokine secretion essential for adaptive immunity activation. This adaptable framework supports combinatorial immunogen integration, facilitating polyvalent therapeutic strategies across diverse disease models. The system provides enhanced therapeutic precision, immunogenicity, and safety profiles compared to conventional passive immunization approaches. Customizable development pathways and technical consultation further streamline translational objectives.
We begin by understanding your specific immunotherapy needs, including the target antigen, desired APC subset, and any existing immunotherapy constructs.
We design a targeting module to ensure specific delivery of your chosen immunogen to the desired APCs. This design is crucial for maximizing the effectiveness of the immunotherapy.
After that, the immunogen, specific to your target, links to the targeting module. This link guarantees not only targeting but also activation of the APCs to produce an immune response against the target.
Systematic evaluation of immunogen-targeting assemblies confirms functional specificity through rigorous analysis of APC binding accuracy and activation potential under controlled conditions.
Therapeutic complexes undergo quantitative analysis in established animal models, with functional endpoints including immune effector stimulation, molecular target suppression, and longitudinal survival benefit monitoring.
Experimental outcomes are presented through standardized reporting protocols, featuring mechanistic insights into immunological cascade modulation, pathway inhibition efficacy, and correlative findings from multimodal investigations.
Q1: How does this service enhance APC function to improve immunotherapy outcomes?
A1: Our platform utilizes precision-engineered APC modulation technology that regulates specific signaling pathways critical for immune activation. By optimizing antigen processing and co-stimulatory molecule expression, we enable dendritic cells to present tumor-associated antigens more efficiently. This results in amplified T-cell priming and improved cytotoxic targeting of malignant cells while maintaining immune homeostasis.
Q2: Can this service be adapted for different types of immunotherapies or target diseases?
A2: Absolutely. The platform's adaptable framework supports customization across multiple immunotherapy modalities. Our team works closely with researchers to reconfigure functional components based on specific disease targets - whether solid tumors, hematologic malignancies, or chronic infections - ensuring optimal compatibility with your therapeutic strategy.
Q3: What are the key advantages of this APC-targeted approach compared to other methods?
A3: Three distinct advantages set our technology apart: 1) Unparalleled cell-type specificity through proprietary surface marker targeting; 2) Multi-modal activation combining pattern recognition receptors with cytokine signaling; 3) Dynamic feedback control preventing systemic cytokine release. This coordinated engagement of innate and adaptive immunity enhances treatment durability while minimizing inflammatory sequelae.
Q4: How does this service address potential safety concerns or risks associated with immunotherapy?
A4: Safety engineering is embedded throughout our development process. All constructs incorporate dual suicide switches and auto-regulatory circuits to prevent cytokine storms. Our comprehensive preclinical package includes TCR repertoire analysis, cross-reactivity screening, and cytokine release syndrome modeling. For clinical translation, we provide IND-enabling toxicology support and immune monitoring protocol development.
Creative Biolabs is devoted to offering high-quality solutions and competent assistance. From initial consultation to final data delivery, we work closely together to guarantee your individual objectives and goals are fulfilled with professionalism and great attention to detail. We supply the tools and materials required for R&D success. Please get in touch with us if you want to improve your APC treatment.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION