Are you currently facing challenges in developing highly targeted immunotherapies, overcoming HLA-restricted responses, or engineering exquisitely specific cell therapies? Creative Biolabs' chimeric HLA antibody receptor (CHAR) construction service helps you overcome these limitations and engineer highly specific cell therapies through advanced genetic engineering and cell therapy design, accelerating your drug discovery process.
The landscape of gene therapy, particularly with CAR-T cells, has revolutionized cancer treatment. However, existing therapies often grapple with challenges such as on-target/off-tumor toxicity and the inherent HLA-restriction of T-cell receptors, limiting broader applicability. The necessity for developing novel approaches like CHARs is paramount to specifically target HLA-peptide complexes on diseased cells, broadening therapeutic windows and enabling precision targeting of intracellular antigens, thereby significantly enhancing safety and efficacy in various disease contexts.
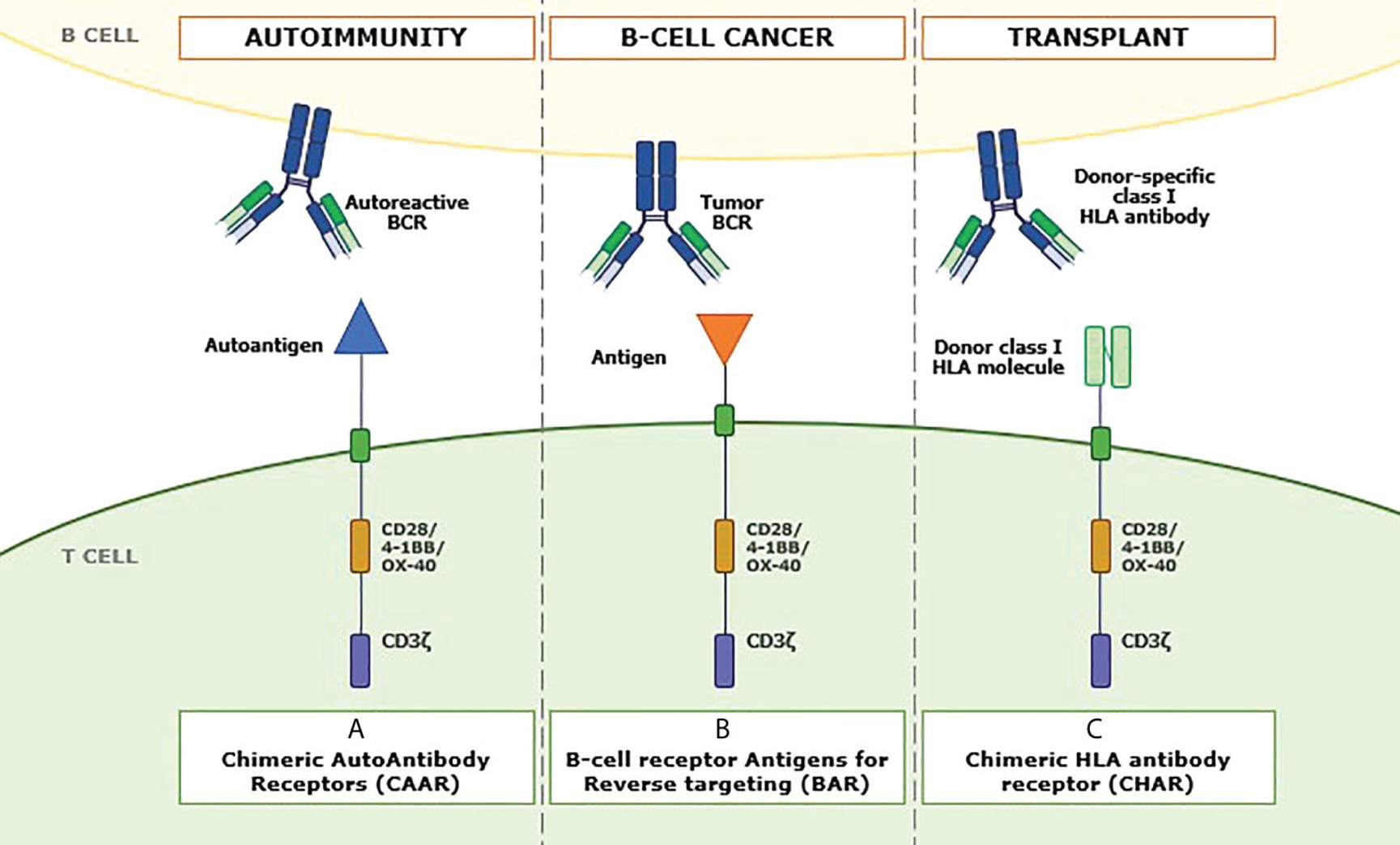 Fig.1 Ligands play a role in antibody-mediated diseases.1
Fig.1 Ligands play a role in antibody-mediated diseases.1
Creative Biolabs' CHAR construction service delivers engineered T-cells precisely targeting intracellular tumor-associated antigens (TAAs) presented by HLA. Enabling highly specific solid tumor immunotherapies, it overcomes traditional CAR-T limitations. Clients receive validated CHAR constructs, expanded T-cell targeting capabilities, and robust preclinical data to accelerate novel cancer therapeutics. We provide expert guidance from target selection to functional validation, leveraging cutting-edge technology for optimal outcomes—enhancing efficacy while minimizing off-target toxicities. Expect:
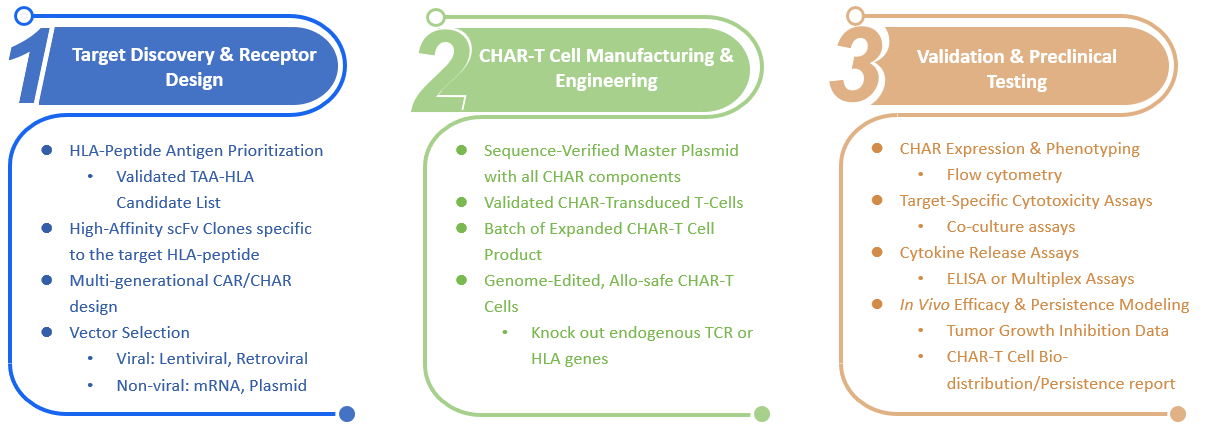
Whether you already have antigen and antibody sequence data or are starting from scratch, our team can guide you through each step - from concept to validated cell product.
Discover How We Can Help - Request a Consultation
To initiate your CHAR construction project, we typically require:
Our expert team analyzes provided target HLA-peptide and antibody variable region sequences. We then design the optimal CHAR construct, integrating the single-chain variable fragment (scFv) to target the specific HLA-peptide complex, a robust transmembrane domain, and intracellular signaling domains for T-cell activation. This design minimizes off-target interactions.
Following the design, the entire CHAR construct is custom-synthesized and cloned into a high-quality lentiviral expression vector. This step ensures high fidelity and prepares the construct for efficient viral packaging and cell transduction.
We proceed to generate high-titer, replication-defective lentiviral particles containing your CHAR construct. Our optimized packaging systems ensure efficient viral production, critical for robust transduction of target cells with minimal cytotoxicity.
Our service includes the transduction of client-provided T cells (or other specified immune cell types) with the generated lentiviral vectors. Following transduction, the CHAR-expressing cells are expanded under optimal conditions to achieve desired cell numbers and viability, ready for your downstream applications.
The final and crucial step involves comprehensive validation of the engineered CHAR-expressing cells. We assess CHAR expression levels via flow cytometry, evaluate the CHAR's binding affinity to the specific HLA-peptide target, and perform in vitro functional assays (e.g., cytotoxicity assays against target cells, cytokine release assays) to confirm potent and specific immune responses.
Creative Biolabs' CHAR Construction Service offers distinct advantages designed to empower your research and therapeutic development.


Q1: How does Creative Biolabs ensure the specificity and efficacy of the constructed CHARs?
A1: Our commitment to quality is unwavering. Creative Biolabs employs rigorous functional validation protocols, including assessing CHAR expression levels, specific antigen binding affinity, and downstream signaling pathways. We also conduct comprehensive in vitro functional assays, such as cytotoxicity assays against target cells and cytokine release analyses, to confirm potent and specific immune responses. For detailed validation protocols and quality control measures, please inquire with our team.
Q2: Can Creative Biolabs accommodate custom HLA-peptide targets for CHAR construction?
A2: Absolutely! Our Chimeric HLA Antibody Receptor construction service is designed with high customizability in mind. We actively collaborate with our clients to design and engineer CHARs against a wide range of unique HLA-peptide targets, leveraging our extensive experience in antibody engineering and advanced cell therapy design. Share your specific target details with us to discuss a tailored solution that meets your research objectives.
Creative Biolabs' CHAR construction service provides a cutting-edge solution for developing highly specific and potent cell therapies. By leveraging our deep expertise in immunology and genetic engineering, we deliver meticulously designed and validated CHAR constructs that address the critical need for precise targeting, offering enhanced safety and efficacy in challenging disease areas. Partner with us to accelerate your immunotherapy breakthroughs.
Reference
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION