Immune Effector Cell associated Hematotoxicity (ICAHT) Management Solutions
Online Inquiry
Background Service Highlights FAQs Contact Us
Background
Immune effector cell (IEC)-based therapies have transformed the treatment of hematologic malignancies and are gaining traction in solid tumor research. However, their potent activity is frequently accompanied by hematopoietic complications—specifically, Immune Effector Cell-associated Hematotoxicity (ICAHT). This phenomenon is characterized by delayed, prolonged, or biphasic cytopenias that extend beyond initial conditioning regimens, suggesting a direct impact of IECs on hematopoiesis.
ICAHT involves multifaceted biological mechanisms, including immune-mediated suppression of hematopoietic stem and progenitor cells (HSPCs), disruption of the bone marrow stromal niche, aberrant cytokine signaling, and metabolic or oxidative stress imposed by activated effector cells. These toxicities pose a major challenge to the optimization of IEC therapies, limiting re-dosing schedules, increasing risk of infections, and hindering patient recovery in clinical scenarios. From a research perspective, deciphering the mechanisms of hematopoietic dysfunction is essential for developing next-generation constructs with reduced off-target effects and enhanced safety profiles.
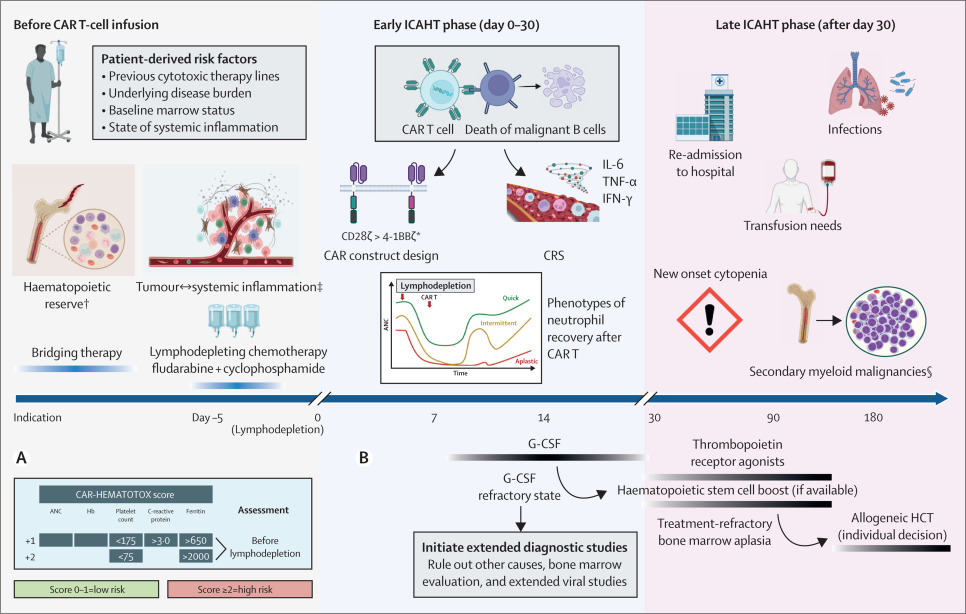 Fig.1 CAR T Cell Therapy Hematologic Toxicity Schedule.1
Fig.1 CAR T Cell Therapy Hematologic Toxicity Schedule.1
To address this unmet need, Creative Biolabs offers a comprehensive ICAHT Management Solutions platform tailored to preclinical, translational, and exploratory research needs. This platform integrates advanced immunobiological modeling, hematopoietic functional assays, and cellular interaction profiling to support systematic investigation of IEC-induced hematotoxicity in a research-use-only context.
Featured Platforms & Specialized Services at Creative Biolabs
Creative Biolabs' ICAHT Management Solutions platform provides a specialized, modular suite of services tailored to the in-depth study of hematopoietic toxicity induced by immune effector cells (IECs). Designed for research-use-only applications, this platform enables scientists to dissect cellular and molecular mechanisms underlying hematopoietic suppression, evaluate effector cell–blood cell interactions, and explore mitigation strategies in a preclinical setting. Leveraging integrated co-culture models, stem/progenitor cell assays, and high-dimensional profiling techniques, we offer comprehensive, construct-agnostic solutions adaptable to various IEC formats including CAR-T, CAR-NK, and TCR-engineered cells.
This module focuses on unraveling the mechanistic basis of hematopoietic dysfunction induced by IECs. Using advanced in vitro and ex vivo models, we simulate immune effector exposure to hematopoietic stem and progenitor cells (HSPCs) and stromal niche components. Assays are designed to characterize the impact of direct cytotoxic signaling, inflammatory stress, and metabolic imbalance on the proliferation, differentiation, and viability of key blood cell precursors. Key technologies include colony-forming unit (CFU) assays, apoptosis and senescence profiling, RNA-seq-based transcriptional analysis, and quantitative cytokine panels. This service is ideal for researchers aiming to assess hematopoietic safety profiles of novel constructs, uncover hematotoxic molecular signatures, and explore protective interventions that can preserve bone marrow functionality.
This module investigates the dynamic interplay between immune effector cells and hematopoietic targets, emphasizing both contact-dependent and soluble factor-mediated mechanisms of suppression. We provide customizable co-culture platforms where IECs are evaluated for their influence on CD34⁺ HSPCs, lineage-committed progenitors, or stromal support cells. By combining flow cytometry-based immunophenotyping, real-time imaging, cytotoxicity assays, and cytokine multiplexing, we enable a high-resolution view of how immune cells modulate hematopoietic cell survival and function. Researchers can also assess the effect of specific signaling domains, co-stimulatory profiles, or activation states on hematopoietic toxicity, making this service an effective tool for comparative construct analysis and early safety de-risking.
Why Choose Us
Mechanistic Depth
The platform moves beyond surface-level toxicity detection by investigating root biological causes of hematopoietic impairment, including transcriptional reprogramming, senescence induction, and lineage differentiation blockade.
IEC-Hematopoietic Interactome Mapping
Enables dynamic analysis of cell-cell communication between immune effectors and hematopoietic cells, leveraging contact-based suppression assays and cytokine-neutralization strategies.
Niche-Mimetic Modeling
Supports co-culture of IECs with stromal niche components such as MSCs, endothelial cells, and osteoblasts to study microenvironmental remodeling and feedback disruption.
Custom Construct Evaluation
Allows quantitative assessment of hematotoxic potential across IEC constructs with varied signaling domains (e.g., 4-1BB, CD28), co-stimulation profiles, or target specificities.
Advanced Profiling Technologies
Integration with multi-omics platforms including bulk RNA-Seq, single-cell transcriptomics, and targeted proteomics for pathway deconvolution and signature identification.
Mitigation Strategy Testing
Compatible with therapeutic candidate screening such as anti-inflammatory agents, immune modulators, or niche protectants to assess reversal of hematopoietic suppression.
FAQs
Q1: Can I evaluate the hematotoxicity of my novel CAR-T design using your platform?
A1: Yes. We offer side-by-side comparison of constructs in hematopoietic suppression models and can incorporate both direct and indirect toxicity assays.
Q2: Do you provide bioinformatics support for omics data?
A2: Yes. All multi-omics services are delivered with complete bioinformatics interpretation and optional publication-ready figures.
Q3: Is it possible to run long-term hematopoietic recovery models?
A3: Yes. We offer extended culture formats to track delayed or biphasic suppression and recovery kinetics.
Contact Us
At Creative Biolabs, we believe that comprehensive hematotoxicity assessment is essential for the safe and effective translation of immune effector cell therapies. Our ICAHT Management Solutions platform provides not only technical execution but also strategic insight into construct refinement and toxicity mitigation.
We welcome collaboration with academic labs, biotech innovators, and pharmaceutical R&D teams seeking to explore hematopoietic safety from a mechanistic and functional standpoint. Whether you are designing your first CAR construct or optimizing a next-generation immune engager, our team is ready to support your program with tailored, publication-grade research solutions.
Reference
-
Rejeski, Kai et al. "Immune effector cell-associated haematotoxicity after CAR T-cell therapy: from mechanism to management." The Lancet. Haematology vol. 11,6 (2024): e459-e470. DOI: 10.1016/S2352-3026(24)00077-2. Distributed under Open Access License CC BY 4.0, without modification.




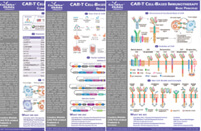


 Fig.1 CAR T Cell Therapy Hematologic Toxicity Schedule.1
Fig.1 CAR T Cell Therapy Hematologic Toxicity Schedule.1




