Are extended timelines in therapeutic development delaying the delivery of essential medicines, compounded by persistent hurdles in antibody engineering such as suboptimal target specificity and binding efficiency? Concurrently, do multifaceted clinical trial demands, including prolonged participant enrollment and labor-intensive data interpretation, further hinder progress? Our AI-driven TCR single-cell screening service addresses these challenges by accelerating drug discovery through an integrated high-throughput platform. This technology enables simultaneous single-cell transcriptomic profiling and functional evaluation, streamlining the identification of viable candidates with precision.
The precise identification and functional investigation of T-cell receptors (TCRs) that bind disease-linked antigens will be absolutely essential for the development of powerful immunotherapies. Although fundamental, conventional methods for TCR discovery are generally time-consuming and resource-intensive. Artificial intelligence (AI) and single-cell sequencing systems have recently advanced by means of their convergence to enable high-throughput detection of TCR-antigen interactions with improved resolution. This combined strategy not only avoids inefficiencies inherent in traditional methods but also simplifies the implementation of TCR-based therapy options into clinical settings.
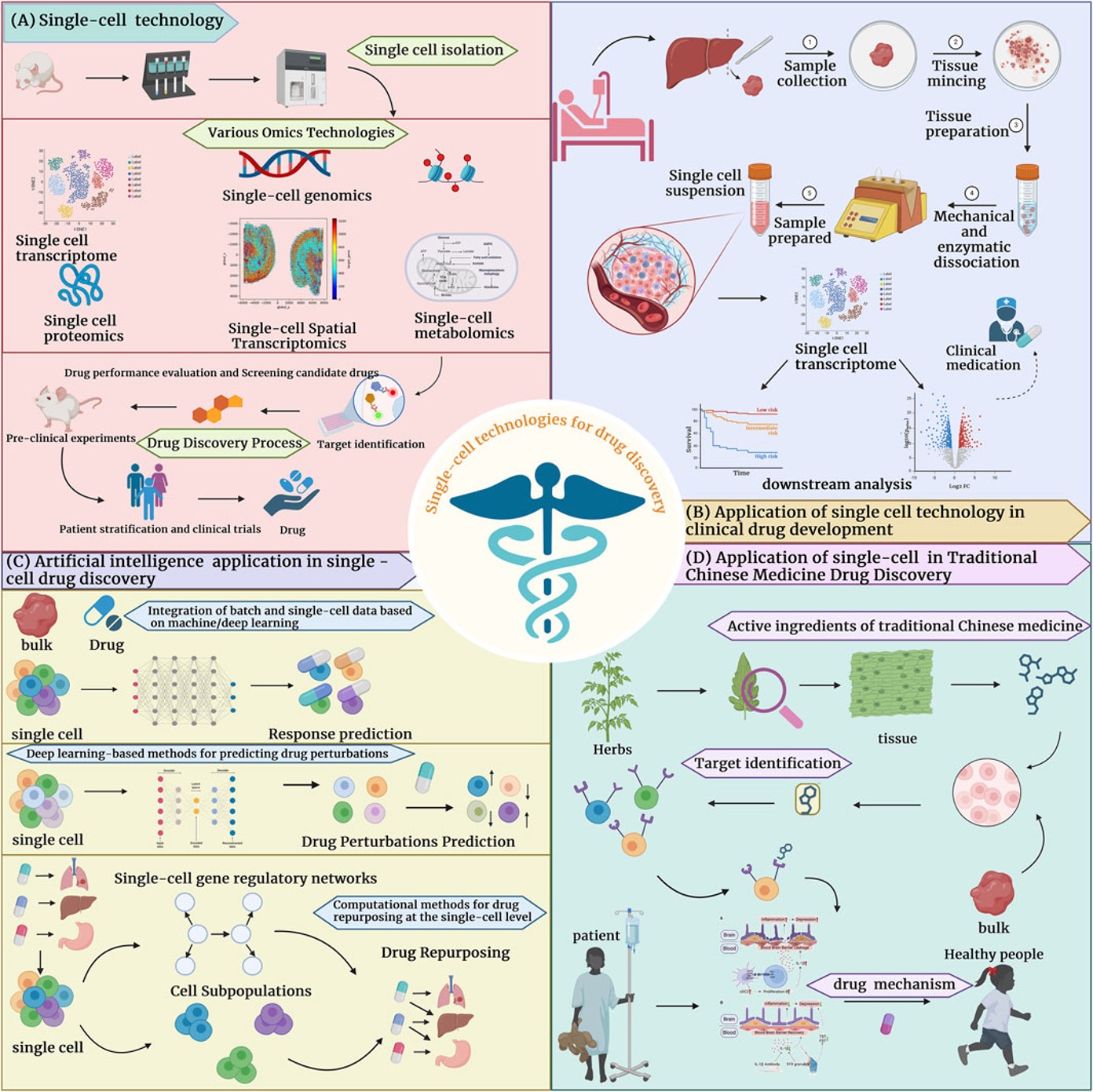 Fig.1 Single-cell technology is widely applied in drug development.1,3
Fig.1 Single-cell technology is widely applied in drug development.1,3
Creative Biolabs has engineered an AI-integrated TCR single-cell screening platform designed to address key challenges in immunotherapy development. This system employs machine learning algorithms to analyze high-dimensional single-cell datasets, enabling rapid identification of antigen-specific TCR sequences with therapeutic potential. By integrating multi-omic profiling that combines V(D)J sequencing with functional readouts, the platform delivers mechanistic insights into TCR-pMHC interactions and clonal behavior. The modular architecture supports tailored experimental configurations for both novel target discovery and therapeutic optimization pipelines, enhancing research outcomes across diverse immunotherapy applications. This methodology streamlines the transition from TCR characterization to clinical candidate selection through standardized analytical frameworks.
We initiate the process by obtaining relevant biological samples, such as patient tumor biopsies, PBMCs, or other T-cell-rich tissues, depending on the therapeutic target and disease.
Step 2: TCR and Transcriptome Sequencing Library PreparationAfter that, we lyse the T cells to extract RNA, and transcript counting during reverse transcription, boost TCR alpha/beta (and occasionally other) chains, usually do whole transcriptome amplification alongside TCR sequencing, and then get the resulting cDNA ready for NGS library building.
Step 3: High-Throughput SequencingFollowing library preparations, we match TCR sequences to reference genes to identify CDR3 regions, demultiplex the data to connect reads to specific cells via barcodes, and align any entire transcriptome data to the relevant reference.
Step 4: AI-Driven Data Analysis and TCR CharacterizationUsing artificial intelligence, we group cells with matching TCR CDR3 sequences, predict their antigen specificity through sequence analysis, combine this with gene expression data to grasp function, and rank interesting TCRs depending on target prevalence in pertinent cell populations.
Step 5: Functional ValidationUsing viral transduction, we manufacture interesting TCR candidates and present them into T cells. We next evaluate their antigen specificity, cytotoxicity, cytokine generation, and other effector activities against target cells by running these modified T cells through in vitro functional tests. Promising in vitro activity candidates could subsequently be tested in preclinical in vivo models to evaluate their pharmacokinetics, safety, and effectiveness.
Summary: A large-scale analysis of over 4.7 billion TCR sequences from COVID-19 patients and healthy donors reveals key signatures of antiviral immunity. This study identifies convergent CDR3 gene usages, specificity groups, and sequence patterns associated with COVID-19 severity. The research demonstrates that T cell clonal expansion correlates with increased effector function and signaling pathways, and that machine learning can accurately predict COVID-19 infection based on TCR sequence features, providing a systems-level understanding of T cell responses to the virus.
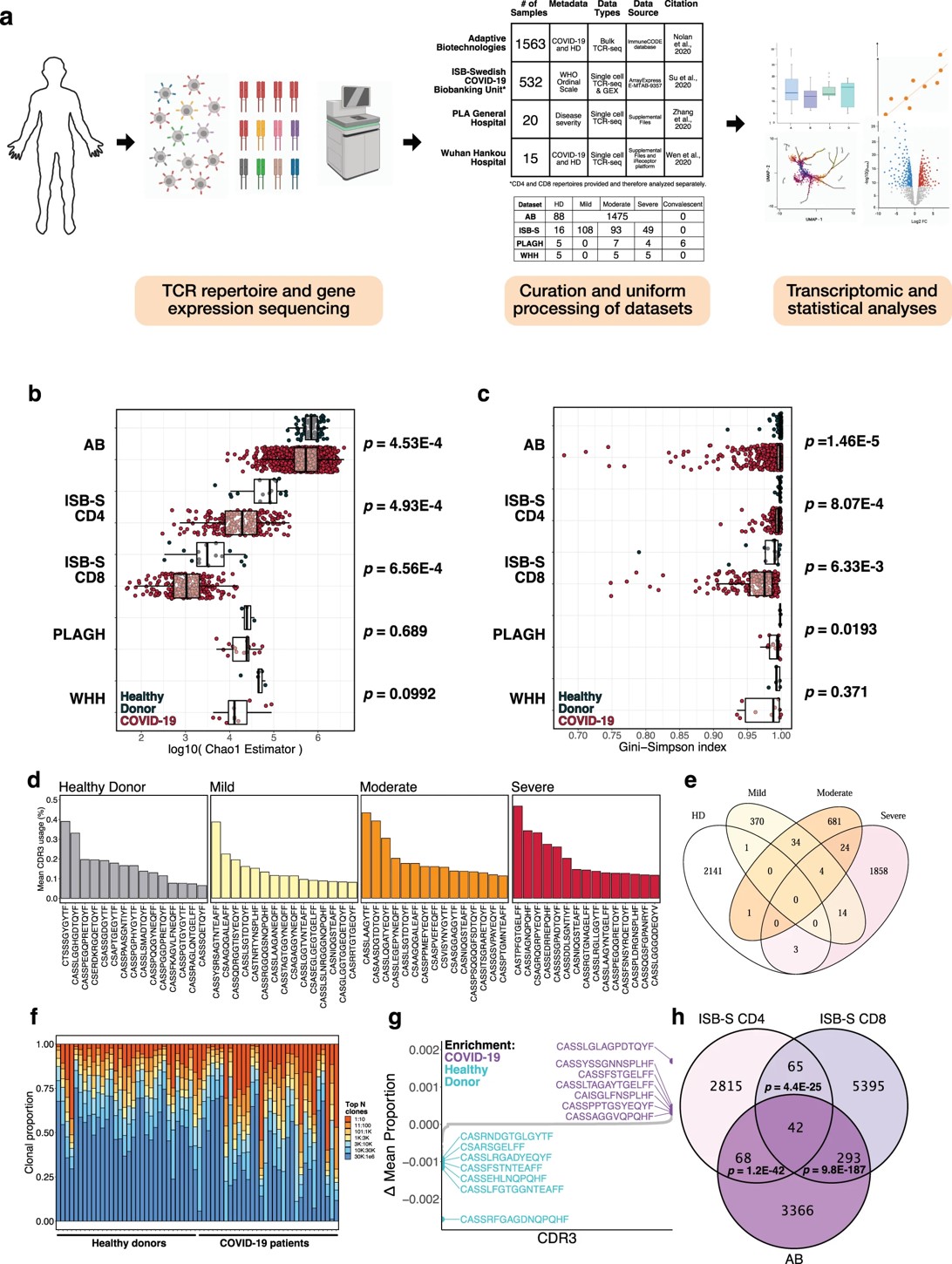 Fig.2 TCR repertoires from COVID-19 patients and healthy donors show patterns in CDR3 gene utilization and diversity.2,3
Fig.2 TCR repertoires from COVID-19 patients and healthy donors show patterns in CDR3 gene utilization and diversity.2,3
Q1: What types of samples are suitable for this service?
A1: We can process a variety of samples, including PBMCs, tumor tissues, and other relevant cell populations.
Q2: How long does the process take?
A2: The typical project duration is 6-12 weeks, but it can vary depending on the project's complexity.
Q3: Can you help with downstream functional validation?
A3: Yes, we offer optional functional validation assays. Inquire for more details on these additional services.
Q4: What kind of data will I receive?
A4: You will receive a comprehensive report, including detailed sequencing data, analysis results, and a list of candidate TCRs.
Q5: How does your AI technology improve TCR screening?
A5: Our AI algorithms enable high-throughput analysis, faster identification of target-specific TCRs, and enhanced specificity.
Creative Biolabs is committed to providing you with the highest quality TCR discovery services. Our AI-driven TCR Single-Cell Screening Service offers a powerful solution for accelerating your immunotherapy research and drug development. If you are interested in our AI-driven TCR single-cell screening service, please feel free to reach out to us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION