Precision Dendritic Cell (DC) Vaccine Development Services: Targeting Tumor with Smart Drug Delivery
Online Inquiry
Background Service Workflow Service Packages Advantages FAQ Why Choose Us
To address the challenges in cancer immunotherapy, such as limited clinical effectiveness and complex antigen/therapeutic delivery, Creative Biolabs provides cost-effective precision dendritic cell (DC) vaccine development services. We help you accelerate the development of highly specific and effective anti-tumor immune responses through advanced DC engineering and innovative smart drug delivery technologies.
The Imperative for Precision DC Vaccines
Dendritic cells (DCs) are pivotal antigen-presenting cells that orchestrate robust anti-tumor immune responses. Despite their potential, conventional DC vaccines have shown modest clinical efficacy, often hampered by issues like inefficient antigen presentation, poor DC migration, and the immunosuppressive tumor microenvironment. Developing precision DC vaccines with smart drug delivery is crucial to overcome these limitations, enabling targeted antigen delivery and enhanced immune activation for more effective cancer treatment, leveraging advancements in gene therapy and nanotechnology.
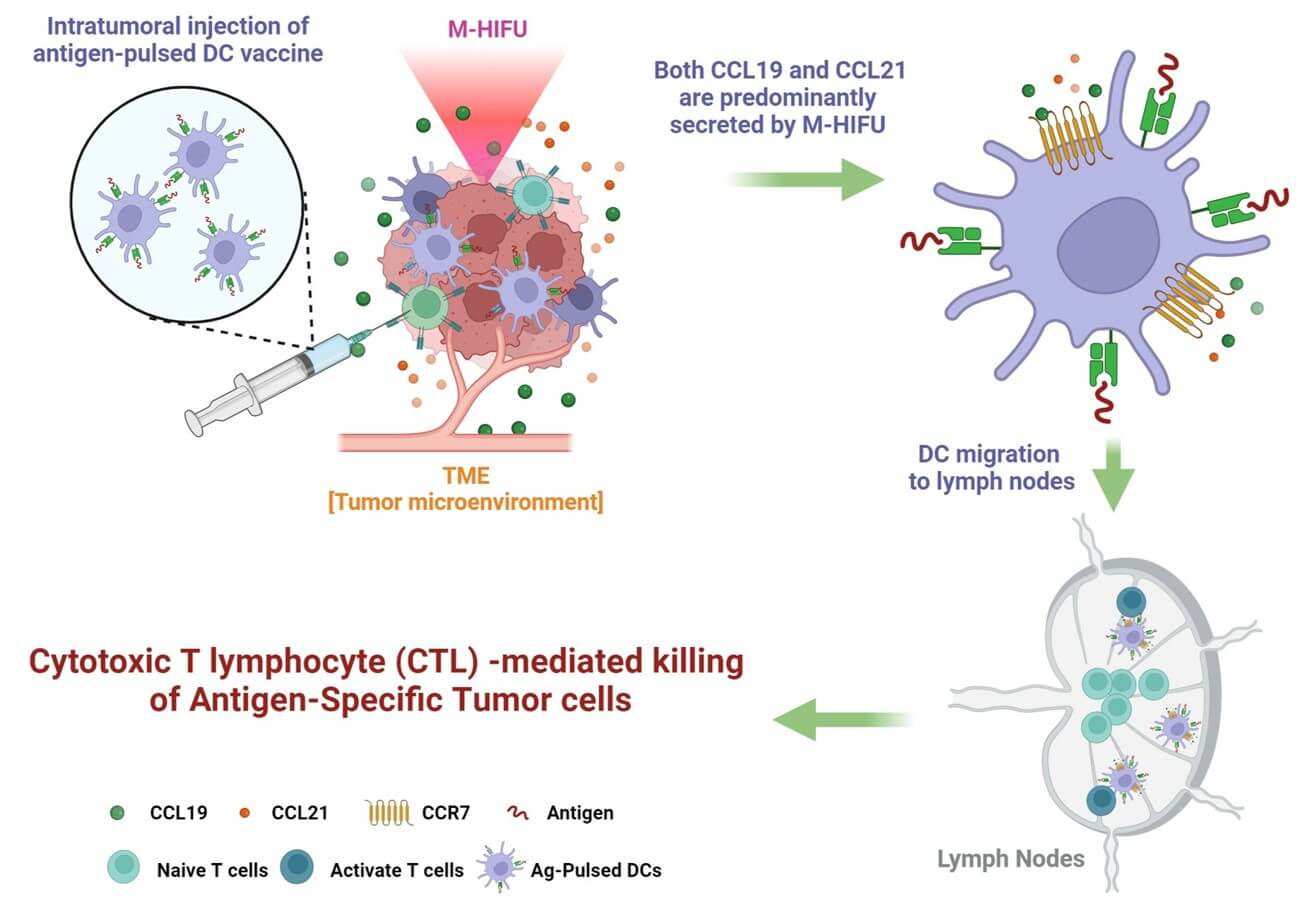 Fig.1 DC vaccine injection and function process.1
Fig.1 DC vaccine injection and function process.1
Our Precision Dendritic Cell (DC) Vaccine Development Services: Targeting Tumor with Smart Drug Delivery
Creative Biolabs' Precision DC Vaccine Development Services provide comprehensive solutions to empower your cancer immunotherapy projects. We deliver tailored strategies designed to elicit potent, specific anti-tumor immunity. You can expect optimized DC vaccine constructs, robust in vitro validation data, and detailed in vivo efficacy reports. Our meticulous quality assurance guarantees the integrity and functionality of every component, ensuring reliable and reproducible results.
How to Work
We utilize advanced bioinformatics to identify optimal TAAs or neoantigens. Antigens are then synthesized as peptides, recombinant proteins, or prepared as genetic constructs (DNA/RNA) for subsequent loading onto DCs. The outcome is highly specific and immunogenic antigen material ready for DC pulsing or transfection.
DCs are differentiated ex vivo from patient-derived PBMCs or chosen cell lines. We employ proprietary protocols to ensure high yield and purity of immature DCs. This step yields a population of highly viable and antigen-responsive immature DCs.
Antigens are loaded onto DCs via various methods including pulsing with peptides/proteins, electroporation of nucleic acids, or conjugation with smart delivery systems. Subsequently, DCs are matured using optimized cocktails to enhance their antigen-presenting and co-stimulatory capabilities. The expected outcome is a population of mature, antigen-loaded DCs with potent immune stimulatory properties.
For enhanced targeting and delivery, antigens or DC-stimulating agents can be encapsulated within or conjugated to smart delivery vehicles, such as peptide-conjugated PAMAM dendrimers. This innovative approach ensures precise delivery to DCs in vivo or enhances ex vivo loading efficiency. This step yields DCs armed with enhanced targeting capabilities and improved immunogenicity.
Rigorous quality control checks are performed at each stage, including cell viability, phenotype (expression of MHC and co-stimulatory molecules), antigen uptake, and cytokine secretion. Final DC vaccine products undergo extensive in vitro functional assays, such as T-cell proliferation and cytokine profiling, to confirm immune activation potential. This ensures a high-quality, functional DC vaccine product ready for in vivo studies or clinical application.
The formulated DC vaccine is tested in relevant animal models (e.g., syngeneic tumor models) to assess its ability to induce anti-tumor responses, inhibit tumor growth, and establish immunological memory. This provides crucial data on the therapeutic potential and safety of the vaccine.
Our Service Profiles
Our Precision DC Vaccine Development Services are broadly categorized into two main profiles, each designed to meet distinct project needs and therapeutic strategies.
This service focuses on the ex vivo manipulation of dendritic cells. We specialize in isolating, expanding, loading, and maturing DCs with tumor antigens outside the body, ensuring optimal antigen presentation and robust T-cell activation. This includes fine-tuning maturation cocktails and antigen loading strategies to maximize vaccine potency before administration.
This profile emphasizes the design and development of smart delivery systems, such as peptide-conjugated PAMAM dendrimers, to precisely target and activate dendritic cells in vivo. We focus on developing carriers that efficiently deliver antigens or immune-modulating agents directly to DCs within the patient, enhancing their ability to initiate and sustain anti-tumor immune responses, thereby overcoming challenges associated with traditional ex vivo approaches.
Our Service Advantages
-
Targeted Efficacy: Specifically targets tumor antigens, maximizing immune response while minimizing off-target effects.
-
Accelerated Development: Our approach expedites the transition from discovery to therapy by enhancing DC potency and improving antigen delivery through smart carriers like dendrimers.
-
Robust Immunity: Provides a robust platform for generating long-lasting anti-tumor immunity.
-
Enhanced Success Rates: Leads to higher success rates in preclinical and potentially clinical stages.
-
Cost & Time Efficiency: Significantly reduces development timelines and costs.
FAQ
Q: What materials do I need to provide to start a Precision DC Vaccine Development project with Creative Biolabs?
A: To initiate your project, we typically require:
-
Tumor-Associated Antigens (TAAs) or Neoantigen Sequences: This includes specific peptide or protein sequences, or genetic information (DNA/RNA) for your target antigens.
-
Patient-Derived Cells (Optional): If your project involves autologous DC vaccines, we'd need patient leukapheresis material or peripheral blood mononuclear cells (PBMCs) for DC isolation.
-
Project Specifications: Detailed objectives, desired immune outcomes, and any specific preferences for smart delivery systems, such as dendrimers.
Why Choose Us
Creative Biolabs is dedicated to accelerating your breakthroughs in cancer immunotherapy. Our Precision DC Vaccine Development Services, enhanced by smart drug delivery technologies, offer a powerful and precise approach to addressing the unmet needs in oncology. Partner with us to leverage our extensive expertise, state-of-the-art platforms, and commitment to scientific excellence to drive your project towards success. For more details about our precision DC vaccine development services, please don't hesitate to get in touch with us.
Reference
-
Baek, Bum-Seo et al. "HIFU-CCL19/21 Axis Enhances Dendritic Cell Vaccine Efficacy in the Tumor Microenvironment." Pharmaceutics vol. 17,1 65. 6 Jan. 2025, https://doi.org/10.3390/pharmaceutics17010065. Distributed under Open Access License CC BY 4.0, without modification.






 Fig.1 DC vaccine injection and function process.1
Fig.1 DC vaccine injection and function process.1




