Using multiplexed detection systems, MSD electrochemiluminescence, and ultra-sensitive ELISA, this platform captures the full spectrum of proinflammatory and regulatory cytokines associated with IEC-HS (e.g., IFN-γ, IL-18, CXCL9, TNF-α).
IEC-HS (Immune Effector Cell–associated Hemophagocytic Lymphohistiocytosis-like Syndrome) is a systemic hyperinflammatory condition that emerges in the context of cellular immunotherapies, particularly CAR-T cell treatments. Although IEC-HS shares overlapping features with cytokine release syndrome (CRS), such as fever and elevated inflammatory markers, it is characterized by deeper immune dysregulation. This includes excessive interferon-gamma (IFN-γ) signaling, macrophage overactivation, cytopenias, and disruption of tissue homeostasis.
Given the subtle presentation and potential for overlap with other immune-related syndromes, robust experimental tools are essential for identifying early-stage indicators, dissecting cellular contributors, and exploring potential mitigation strategies. Creative Biolabs has developed a comprehensive, modular IEC-HS Management Solution family, integrating cutting-edge assays across cytokine analysis, immune phenotyping, genomics, and biomarker discovery.
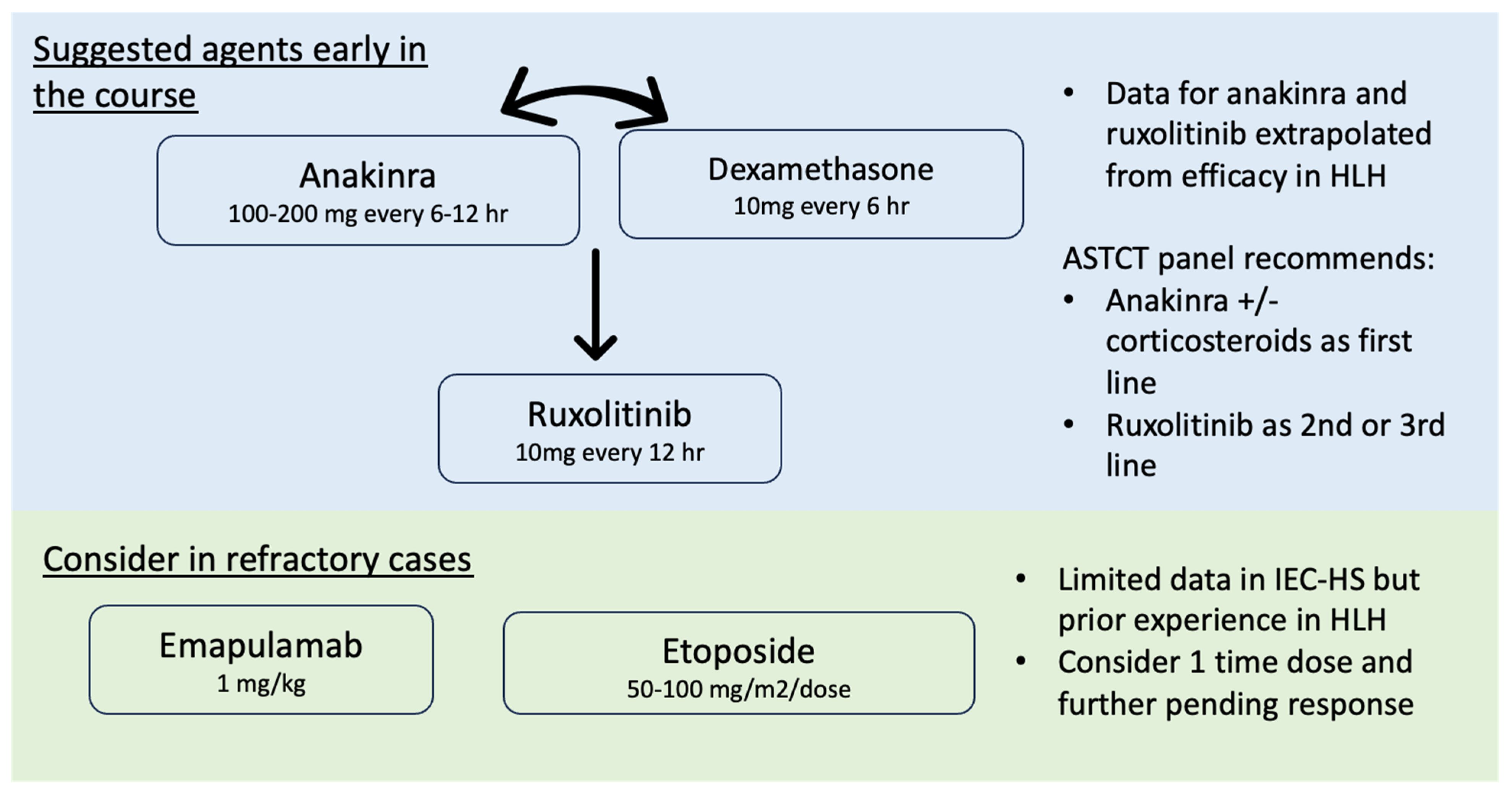 Fig.1 IEC-HS treatment regimen.1
Fig.1 IEC-HS treatment regimen.1
Our IEC-HS platform includes the following four highly specialized services, designed to interrogate immune activation patterns, systemic dysregulation, and underlying triggers of HLH-like syndromes in preclinical CAR-T studies:
Using multiplexed detection systems, MSD electrochemiluminescence, and ultra-sensitive ELISA, this platform captures the full spectrum of proinflammatory and regulatory cytokines associated with IEC-HS (e.g., IFN-γ, IL-18, CXCL9, TNF-α).
High-dimensional flow cytometry and CyTOF-based analyses enable deep characterization of immune cell subsets, including activated monocytes, T cells, NK cells, and regulatory macrophages.
NGS-based approaches are applied to uncover genetic predispositions and expression signatures involved in IEC-HS development.
By integrating transcriptomic (RNA-seq), proteomic (LC-MS/MS), and metabolomic (NMR, MS) data, this platform enables systematic biomarker discovery and pathway modeling.
Includes interferon-inducible genes (e.g., CXCL9, CXCL10), ferritin, sCD163, and immune checkpoint markers known to be dysregulated in HLH-like syndromes.
Supports cross-validation of molecular, cellular, and secreted signatures through a single sample processing pipeline.
Validated across human PBMCs, mouse models, and humanized systems relevant to CAR-T preclinical studies.
Allows researchers to tailor marker sets and detection thresholds based on specific immunotherapy designs or in vivo protocols.
Close consultation with immunologists and CAR-T specialists ensures optimal experimental design and interpretation.
Q1: Can the cytokine panel distinguish between IEC-HS and conventional CRS?
A1: Yes. CRS typically involves IL-6, IL-10, and TNF-α, while IEC-HS exhibits elevated levels of IFN-γ, IL-18, CXCL9, and soluble CD163, among others. Our cytokine profiling service includes markers specific to HLH-like inflammatory cascades for accurate differentiation.
Q2: What is the advantage of using your immunophenotyping platform in IEC-HS studies?
A2: We focus on surface and intracellular markers associated with macrophage activation syndrome and lymphocyte dysfunction, allowing for precise identification of aberrant cell states contributing to hyperinflammation.
Q3: Do you provide pathway analysis as part of genomic testing?
A3: Yes. Beyond variant detection, we offer bioinformatics services including differential gene expression analysis, pathway enrichment, and immune cell deconvolution to uncover IEC-HS–relevant mechanisms.
Q4: How customizable is the biomarker discovery workflow?
A4: Highly customizable. You may provide specific sample types, target pathways, or preferred readout methods. We can integrate data from external platforms as well, ensuring compatibility with your ongoing research.
Q5: Are these services applicable to animal models?
A5: Absolutely. Our assays have been adapted for use in mouse, humanized mouse, and non-human primate models. We also offer cross-species reagents for comparative studies.
At Creative Biolabs, we believe in building strong scientific collaborations that drive innovation. Whether you're exploring IEC-HS as part of a larger CAR-T safety evaluation project or seeking to validate new biomarkers, we're here to support you. Our platform is not only modular and customizable, but also backed by a team of immunologists, molecular biologists, and bioinformaticians who understand the complexities of immunotoxicology research.
We welcome partners from biotech, academia, and translational R&D to join us in pushing the boundaries of IEC-HS understanding. Let's accelerate your research with precision data, expert insight, and shared purpose.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION