Cytokine-Induced Killer (CIK) cells represent a promising alternative to conventional CAR-T cell therapies in cancer immunotherapy. Their unique features, including major histocompatibility complex (MHC)-unrestricted cytotoxicity and minimal induction of graft-versus-host disease (GvHD), highlight their potential to address several limitations associated with CAR-T cells. As advances in biotechnology continue, the development and enhancement of CIK therapies have come to the forefront of cancer treatment innovations. Creative Biolabs, a leader in the field with over 20 years of experience, is at the cutting edge of these developments, offering comprehensive services and solutions in CIK therapy enhancement.
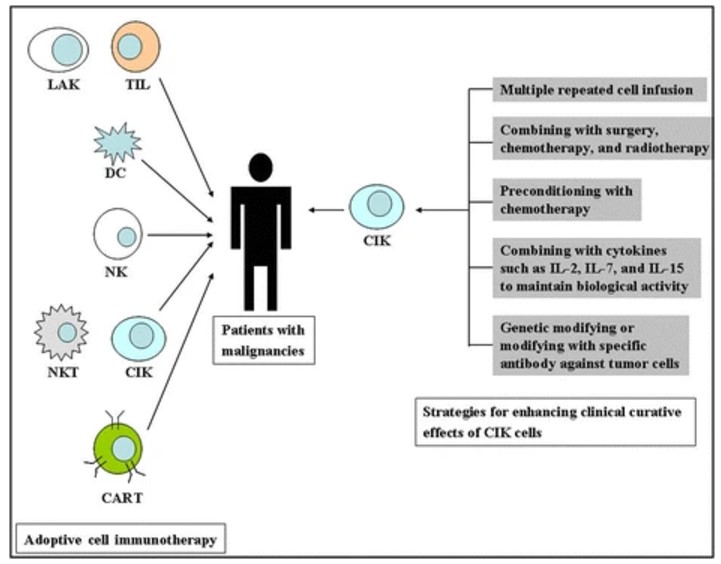 Fig.1 Adoptive cellular immunotherapy and improvement strategies for cytokine-induced killer (CIK) cells.1
Fig.1 Adoptive cellular immunotherapy and improvement strategies for cytokine-induced killer (CIK) cells.1
Creative Biolabs is committed to advancing the therapeutic efficacy of CIK cells through a structured, innovative, and scientifically-grounded approach. By leveraging state-of-the-art technology and a deep understanding of immunology, Creative Biolabs focuses on enhancing the functional capacities of CIK cells. This development is steered by a team of seasoned professionals who integrate various scientific methods to optimize the production and application of CIK cells in clinical settings.
Creative Biolabs offers a variety of featured packages aimed at enhancing the functionality and application of CIK cells, each tailored to meet specific therapeutic needs:
Immune Checkpoint Inhibitor integrated CIK Therapy Improvement Service
This service integrates cutting-edge immunotherapies, including immune checkpoint inhibitors, to enhance the efficacy of CIK cells, aiming to overcome resistance mechanisms in solid and hematologic malignancies.
Antibody Integrated CIK Therapy Improvement Service
This package leverages the power of monoclonal and bispecific antibodies to redirect and amplify the cytotoxic activity of CIK cells in an antigen-specific manner, significantly broadening their therapeutic scope.
Nanomedicine Integrated CIK Therapy Improvement Service
By employing nanotechnology, Creative Biolabs enhances the delivery and targeting of CIK cells, improving their homing capabilities to tumor sites and minimizing off-target effects.
Adjuvant Immune Stimulant Integrated CIK Therapy Improvement Service
This service involves the use of adjuvants to stimulate immune responses, enhancing the proliferation, survival, and cytotoxic activity of CIK cells in the tumor microenvironment.
Combining CIK cells with chimeric antigen receptors (CARs), this service aims to enhance specificity and potencies, such as targeting relapsed and refractory acute lymphoblastic leukemia.
Modified Cytokine Receptor-CIK Development Service
Modification of cytokine receptors in CIK cells to improve their response profiles and ensure robust antitumor activity in various oncological settings.
CIK Incorporating Safety Switch Development Service
Integration of safety switches in CIK cells to allow controlled deactivation, enhancing the safety profile of these therapies.
Dendritic Cell (DC)-CIK Development Service
By utilizing dendritic cells (DCs), this service enhances the antigen-presenting capabilities of CIK cells, improving their activation and therapeutic efficacy.
Our development services provide numerous advantages, manifesting in the elevation of both scientific outcomes and clinical viability:
Q1: What makes CIK therapy a viable alternative to CAR-T therapy?
A1: CIK therapy offers a less toxic, more versatile, and cost-effective approach with a broader range of tumor targeting capabilities, making it an attractive option for patients not eligible for CAR-T therapy.
Q2: Can Creative Biolabs tailor CIK therapy to specific cancers?
A2: Yes, Creative Biolabs uses advanced genetic engineering to tailor CIK cell therapies to target specific cancer antigens, enhancing targeted efficacy and personalization of treatment.
Q3: What are the logistical benefits of using CIK cells over CAR-T cells?
A3: CIK cell therapies are more straightforward to produce at local facilities, avoiding the complex logistics and expenses associated with centralized CAR-T cell production, thus enhancing accessibility for patients.
By emphasizing innovation, collaboration, and precision, Creative Biolabs stands at the forefront of CIK therapy improvement, championing solutions that hold the promise of transforming cancer treatment paradigms worldwide.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION