Chimeric Antigen Receptor T-cell (CART) therapy represents a groundbreaking advancement in cancer immunotherapy. At the core of this therapeutic innovation lies the genetic engineering of T-cells using plasmid DNA vectors. The manufacturing of Good Manufacturing Practice (GMP)-like plasmid DNA is crucial to ensure the safety, efficacy, and regulatory compliance of these therapies. Creative Biolabs, with over two decades of industry experience, excels in providing superior GMP-like plasmid manufacturing services to support CART development.
GMP-like plasmid DNA is designed for pre-clinical studies such as animal testing of drug safety and metabolism. This grade adopts key features of GMP guidelines, ensuring a production process with stringent document control and traceability. Creative Biolabs leverages an advanced, streamlined manufacturing process to produce high-quality plasmid DNA for CART applications. Our facility is equipped with state-of-the-art technologies and adheres to rigorous GMP-like standards to ensure that each batch of plasmid DNA meets stringent quality and safety criteria.
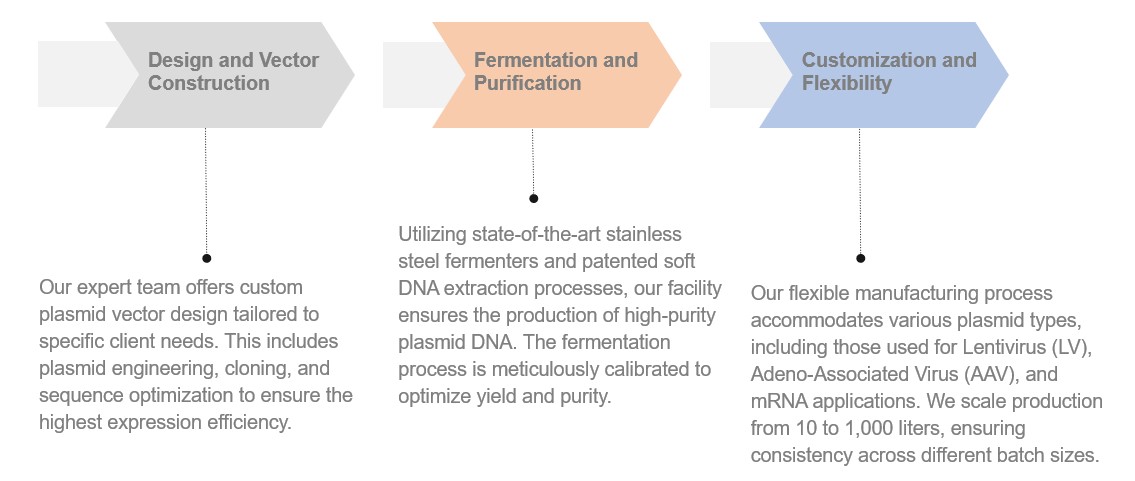
Creative Biolabs provides a comprehensive spectrum of services to support every aspect of plasmid DNA manufacturing:
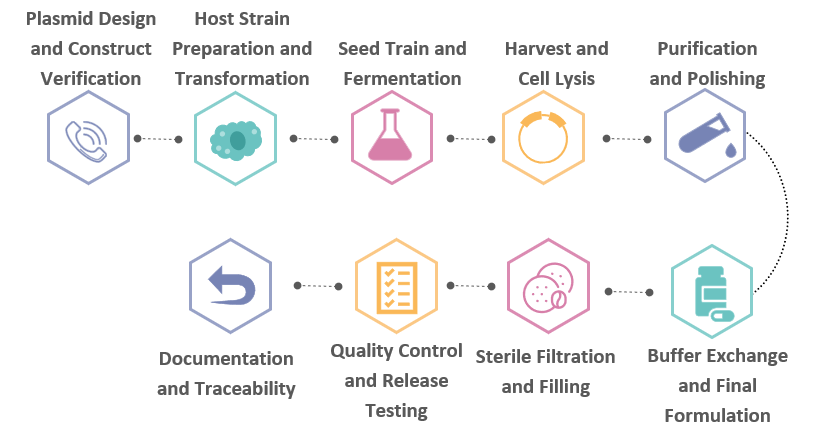
At Creative Biolabs, our GMP-like plasmid DNA manufacturing process operates within a robust, quality-focused framework that incorporates key GMP principles. This ensures high-purity, consistent plasmid outputs suitable for CAR-T research and preclinical use. Every production phase is meticulously engineered and verified to attain outstanding uniformity, traceability, and adherence to regulatory standards.
Contact our team for additional information and to discuss your project needs.
Our GMP-like plasmid manufacturing follows stringent quality assurance protocols to guarantee the safety and efficacy of the final product. Our quality control assays encompass a range of tests to ensure comprehensive quality assessment:
We provide detailed documentation for each production batch, including Certificates of Analysis (CoA), batch records, and traceability reports. These documents ensure full transparency and compliance with regulatory requirements.
All GMP-like CAR-T plasmid DNA products from Creative Biolabs are manufactured under a controlled, validated process and released according to strict internal specifications. Each batch is accompanied by a detailed Certificate of Analysis (CoA) that confirms identity, purity, safety, and functionality.
The following table summarizes typical release specifications for our standard GMP-like plasmid DNA grade. Custom specifications and analytical parameters can be developed upon client request.
| Category | Test Item | Acceptance Criteria | Analytical Method |
|---|---|---|---|
| General Characteristics | Appearance | Clear, colorless solution, no visible particles | Visual inspection |
| Concentration | 1.0–5.0 mg/mL | UV spectrophotometry at 260 nm | |
| OD260/OD280Ratio | 1.8–2.0 | UV spectrophotometry | |
| pH | 7.0–7.5 | pH meter | |
| Identity | Restriction Enzyme Mapping | Pattern matches reference plasmid map | Agarose gel electrophoresis |
| Sequence Verification | 100% identity with reference sequence | Sanger sequencing / NGS | |
| Purity and Topology | Supercoiled DNA Fraction | ≥ 90% (preferably ≥ 95%) | Agarose gel densitometry / HPLC |
| Open Circular (OC) DNA | ≤ 5% | Agarose gel analysis | |
| Linear DNA | ≤ 2% | Agarose gel analysis | |
| Residual Impurities | Residual Host Genomic DNA | ≤ 1% of total nucleic acid | qPCR / PicoGreen assay |
| Residual RNA (ROP) | ≤ 1% | UV absorbance / Bioanalyzer | |
| Residual Host Proteins | ≤ 0.1 µg/mg plasmid DNA | ELISA | |
| Residual Endotoxin | ≤ 0.1 EU/µg DNA | LAL assay | |
| Residual Antibiotic | Not detected | HPLC | |
| Metal Ion Residues | ≤ 10 ppm total metals | ICP-MS | |
| Safety and Sterility | Bioburden | < 10 CFU/100 µL | Plate count |
| Sterility | No growth observed | USP <71> sterility test | |
| Mycoplasma | Negative | PCR-based detection | |
| Functional Quality | Transfection Efficiency | Comparable to the control plasmid | In vitro transfection assay |
| Integrity | No degradation bands | Agarose gel electrophoresis | |
| Stability / Storage | Stability (Real-Time) | ≥ 12 months at −20 °C (validated) | Periodic QC testing |
| Stability (Accelerated) | 1 month at +37 °C: ≥ 90% supercoiled DNA retained | Accelerated stability test |
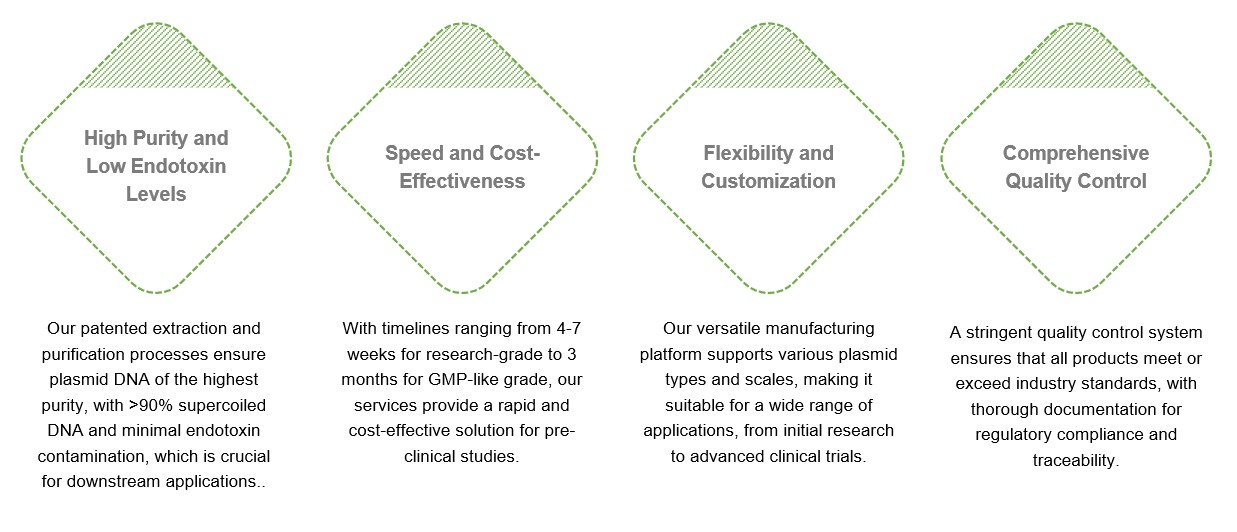
Creative Biolabs' GMP-like plasmid manufacturing service offers several unique advantages:
How does GMP-like manufacturing differ from full GMP?
GMP-like manufacturing incorporates key GMP features such as document control and traceability but is designed to be a more cost-effective and faster alternative for pre-clinical studies. The quality attributes are comparable, but the scale and some production specifics might differ.
What are the endotoxin levels in GMP-like plasmid DNA?
Our GMP-like plasmid DNA has extremely low endotoxin levels, which is critical for maintaining high transfection efficiency and viability in cell lines.
By offering high-quality plasmids produced under meticulous conditions, we empower researchers and clinicians to advance in their efforts to develop innovative and life-saving treatments. Explore the full potential of our GMP-like plasmid manufacturing services and take your CART research and clinical applications to new heights.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION