Creative Biolabs delivers a broad-spectrum GMP-compliant immunocyte production service designed to streamline therapeutic cell product development. By integrating cutting-edge bioprocessing methodologies with stringent quality assurance protocols, this service circumvents critical bottlenecks in manufacturing scalability and protracted development cycles. The platform ensures regulatory-ready cellular substrates, facilitating reproducible translation from preclinical validation to clinical-grade production.
Rapid development in the area of cell therapy offers hope for many different ailments. Nonetheless, transitioning from research applications to therapeutic implementations necessitates rigorous compliance with Good Manufacturing Practices (GMP). GMP compliance guarantees uniformity, safety, and effectiveness of cell therapy products. Overcoming obstacles in cell therapy development, enabling regulatory approvals, and finally providing life-changing medicines to patients depend on strong and consistent GMP-compliant active immunocyte manufacturing services.
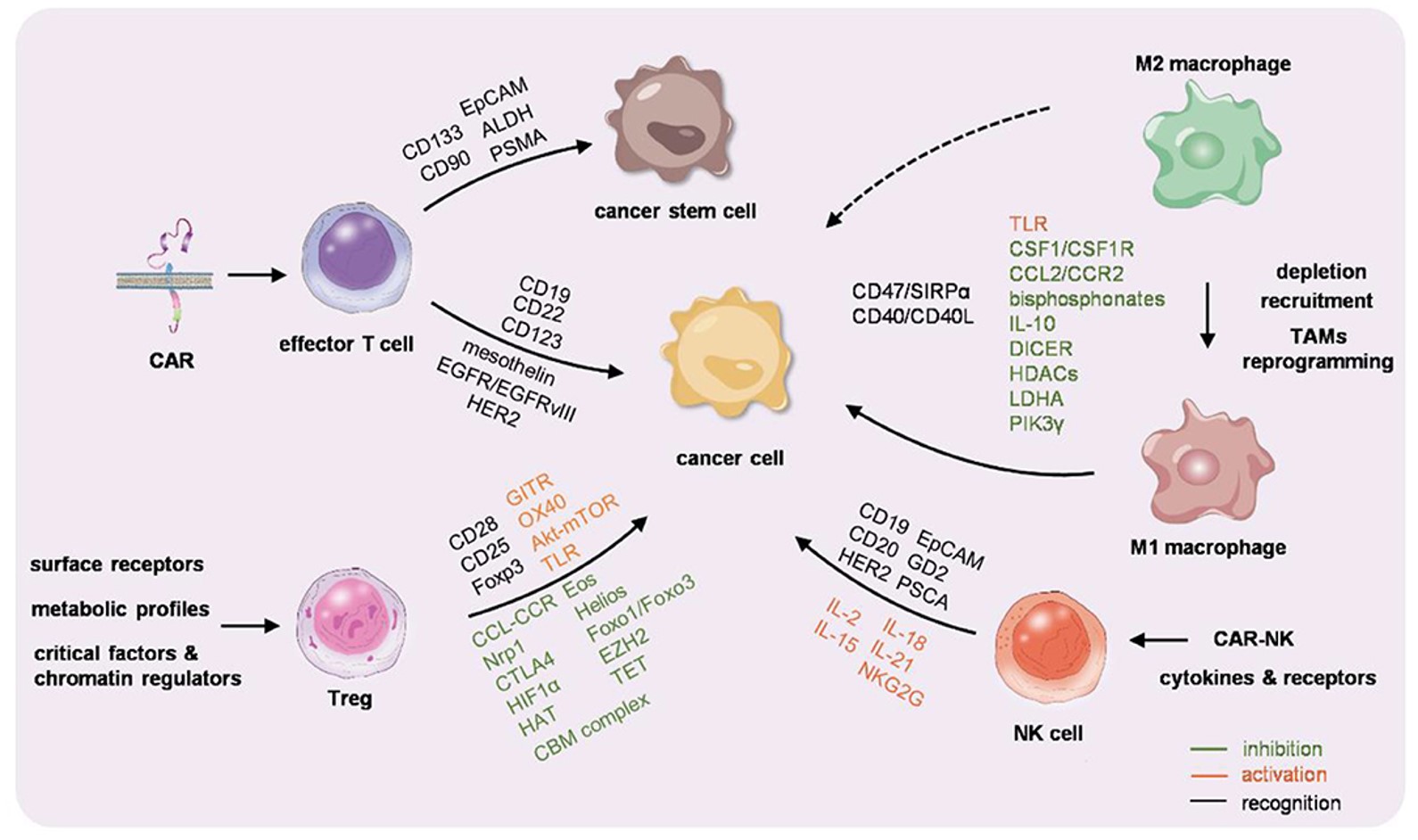 Fig.1 Immunocyte-based cancer therapy.1
Fig.1 Immunocyte-based cancer therapy.1
Creative Biolabs provides a thorough GMP-compliant service for active immunocyte production, aimed at optimizing your cell therapy project. Our offerings encompass comprehensive solutions, starting from the initial consultation phase through to the final delivery of the product, while maintaining the utmost quality and adherence to compliance standards. In detail, our operational process encompasses:
Our process starts with an in-depth consultation to gain a comprehensive understanding of your unique project needs, encompassing the specific immunocytes involved, the target disease, and the intended production scale. We assess the feasibility of your project and provide tailored insights on optimal strategies.
Required Starting Materials: Client-provided cell source (e.g., PBMCs, leukapheresis product), specific growth factors or cytokines, and relevant research protocols.
Our team of specialists will design and refine a comprehensive production process customized to your unique immunocyte type. This encompasses the optimization of cell culture conditions, the selection of suitable reagents, and the establishment of essential quality control parameters.
Key Steps Involved:
Following the strict industry requirements, we produce your active immunocytes in our modern GMP-compliant facility. Our facility features state-of-the-art cell culture systems, specialized cleanrooms, and rigorous quality control laboratories.
Key Steps Involved:
Our quality control professionals test every manufacturing step to make sure the active immunocyte product meets criteria and laws. Every batch's quality and conformance are validated by a Certificate of Analysis (CoA). The GMP-compliant active immunocyte product, batch Certificates of Analysis, and complete manufacturing and quality control records are among the last deliverables.
Our thorough regulatory help simplifies your other regulatory filings as well as your IND application. Using the vast knowledge of our staff, we provide professional advice on GMP compliance, thorough documentation, and successful regulatory agency contacts. Depending on project complexity, the particular immunocyte type, and the required manufacturing size, the usual period for this service is calculated to be 8 to 16 weeks.
Our services can adapt to the GMP production of various active immune cells (listed below) and can be customized to meet the different needs of customers:
Q1: What determines the production timeframe for GMP-grade immunocyte manufacturing?
A1: Production schedules are contingent on cell yield specifications and protocol complexity. Customized timelines are developed through collaborative scoping of client-specific parameters. A detailed project plan is provided upon formal consultation.
Q2: Which quality assurance metrics are applied to finished cellular products?
A2: Rigorous multiparameter assessments—encompassing viability, phenotypic purity, functional potency, microbiological sterility, and genomic identity—are conducted to verify compliance with pharmacopeial standards. Protocol-specific testing frameworks are available upon request.
Q3: How do GMP-certified immunocytes enhance therapeutic development pipelines?
A3: GMP compliance guarantees batch-to-batch repeatability, regulatory-compliant safety profiles, and complete production traceability, hence reducing biological variability and adventitious agent concerns. Achieving strong preclinical validation and enabling regulatory approval in clinical-stage initiatives depends on these qualities.
If you want to get more information about our GMP-compliant active immunocyte production service, please feel free to contact us.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION