Chimeric Antigen Receptor (CAR) T cell therapy represents a revolutionary advancement in the treatment of various malignancies, particularly B cell malignancies. The process involves the genetic engineering of patients' T cells to express CARs, enabling them to specifically target and eradicate cancer cells. The efficient production of high-quality, GMP-grade plasmid DNA is crucial for this therapy's success, serving as the backbone for CAR T cell manufacturing. Creative Biolabs has been at the forefront of this field for over two decades, offering unparalleled expertise in the development and production of GMP plasmid DNA designed for CAR T cell applications.
Creative Biolabs provides comprehensive GMP plasmid DNA manufacturing services crucial for CAR T cell therapy. Our state-of-the-art facilities are equipped to handle the intricate processes involved in producing plasmid DNA that meets stringent global regulatory standards. By leveraging cutting-edge technologies, we ensure the highest levels of purity, potency, and safety in our products. We support the manufacturing process from early-stage development through to commercial-scale production, emphasizing compliance with strict guidelines.
Our GMP plasmid DNA manufacturing services are tailored to support a wide range of applications within CAR T cell therapy, including:
Plasmid DNA Development and Production
We employ advanced techniques for the development and production of plasmid DNA, including high-density E. coli culture technology, to produce gram-scale quantities of GMP-grade plasmid DNA.
Plasmid DNA Development and Production
We develop and prepare MCBs of recombinant E. coli, ensuring the reliability and stability required for large-scale plasmid DNA production.
Scale-Up Capability
Our facilities can produce plasmid DNA in batch sizes ranging from 30 to 2200 liters, accommodating various needs from research-grade to full commercial-scale production.
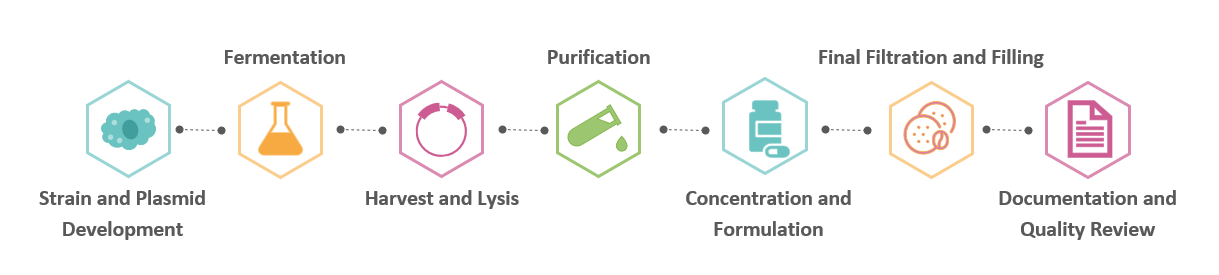
Our plasmid DNA production process follows a standardized, fully traceable procedure designed to ensure consistency, scalability, and conformity to global quality standards. Each stage is closely monitored via in-process checks and documented in accordance with GMP guidelines.
Connect with our team to receive further information and review your project plan.
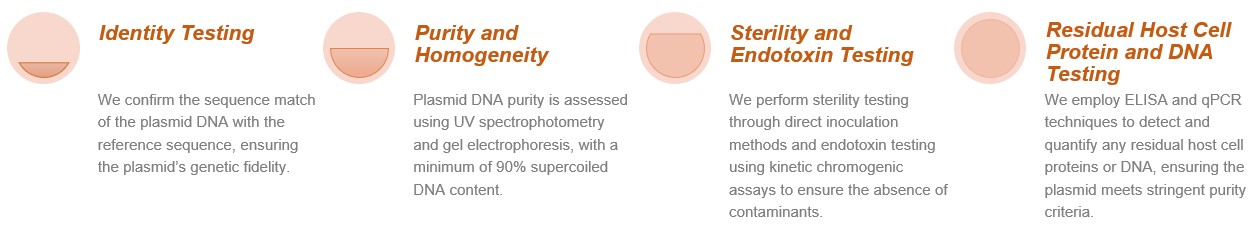
Ensuring the integrity and safety of plasmid DNA is a top priority in our GMP manufacturing process. Our quality control protocol includes extensive in-process and release testing:
Our plasmid DNA manufacturing process is guided by defined performance metrics to ensure consistent quality, safety, and efficiency across every production batch. All parameters are monitored under strict in-process and release criteria to meet global quality expectations.
| Parameter | Description | Typical Specification |
|---|---|---|
| Supercoiled DNA Content | Percentage of plasmid DNA in supercoiled conformation, a critical indicator of functional integrity. | ≥ 85 % |
| Purity | Overall removal of host cell contaminants such as RNA, genomic DNA, and proteins. | ≥ 95 % purity (HPLC or gel-based analysis) |
| Endotoxin Level | Measure of residual endotoxin content to ensure safety for downstream applications. | ≤ 0.1 EU/µg DNA |
| Residual Host DNA | Trace level of chromosomal DNA from production host cells. | Below detection limit |
| Residual RNA/Protein | Verification of complete removal of host-derived biomolecules. | Below detection limit |
| Yield | DNA mass output per production batch, depending on plasmid size and fermentation scale. | Typically 5–10 mg/L to >100 mg/L |
| Batch-to-Batch Consistency | Reproducibility of product quality parameters across multiple runs. | RSD < 10 % |
All performance data are supported by validated analytical methods and documented in the final CoA. These metrics reflect our commitment to reproducible, high-quality plasmid DNA suitable for use in a broad range of advanced biomanufacturing applications. Please contact our team for additional information and to discuss your project requirements.
"We'd struggled with batch variability from other suppliers until switching to Creative Biolabs. Their standardized workflow eliminated that - our last 4 runs had less than 2% variance in yield. When a raw material delay threatened our deadline, they sourced an alternative with full qualification data, keeping us on track. Technical expertise paired with real problem-solving - exactly what we need. Dr. K. P**.
"As a lab scaling from preclinical to Phase I, we needed a plasmid manufacturer that could grow with us. Creative Biolabs did exactly that: starting with 50 mg batches, they smoothly scaled to 500 mg without sacrificing quality. Their in-process endotoxin checks kept our downstream T-cell transduction rates steady, and their team proactively updated us on timeline shifts. Far more reliable than our previous supplier. Prof. M. T**.
Creative Biolabs brings extensive capabilities to the table, ensuring efficient and compliant GMP plasmid DNA production:
Advanced Bioprocessing Technologies
Utilizing state-of-the-art single-use bioreactor systems, we eliminate cross-contamination risks and optimize plasmid yield and purity.
End-to-End Manufacturing Solutions
From cell line creation, plasmid production, and purification to final product packaging, our integrated approach streamlines the manufacturing process, reducing turnaround times.
Robust Compliance and Documentation
Our processes adhere to GMP guidelines, with thorough documentation and traceability maintained throughout the production cycle. Certificates of Analysis (COAs) and comprehensive batch records are provided for each lot.
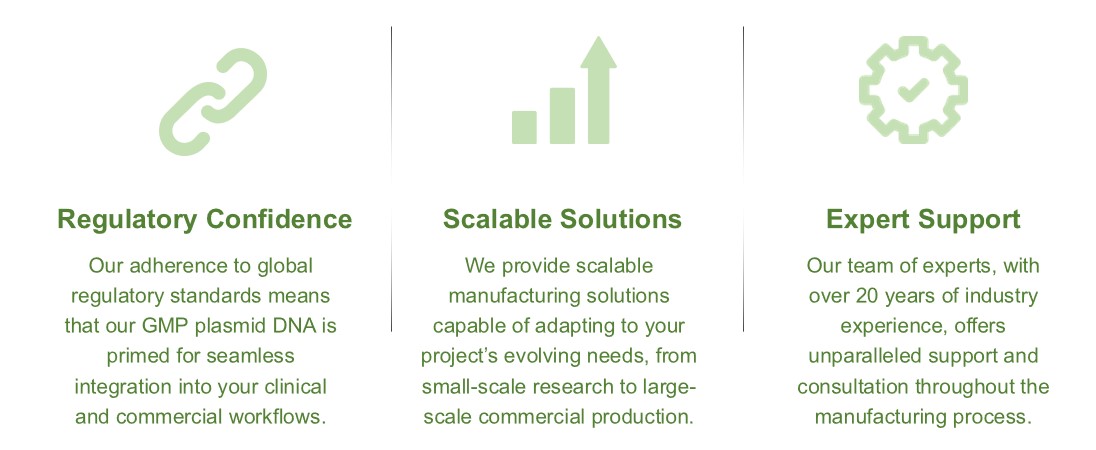
Partnering with Creative Biolabs offers multiple benefits for your CAR T cell therapy program:
What types of plasmid DNA do you produce?
We produce several grades of plasmid DNA, including research-grade, GMP-like, and full GMP-grade plasmids, tailored to different stages of drug development and clinical trials.
How do you handle regulatory compliance?
All our processes comply with strict guidelines, supported by thorough documentation and traceability to meet global regulatory requirements.
Creative Biolabs is committed to advancing the field of CAR T cell therapy through the provision of high-quality, GMP-grade plasmid DNA. By ensuring the highest standards of production and quality control, we empower our clients to bring innovative therapies to market, improving patient outcomes worldwide.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION