Creative Biolabs provides a rapid plant-based antibody production service that solves issues in long antibody development timelines, difficulties in achieving high-yield antibody production, and the need for cost-effective manufacturing, thereby accelerating your research and development, obtaining high-quality antibodies, and streamlining your production process.
Leveraging plants as bioreactors, plant-based antibody generation has become a viable substitute for conventional mammalian cell culture methods. Among numerous benefits this method provides are scalability, economy, and less chance of mammalian pathogen infection. Extensive research has helped to promote the development of plant-based antibody manufacture by proving its ability to generate a range of antibodies for both therapeutic and diagnostic uses.
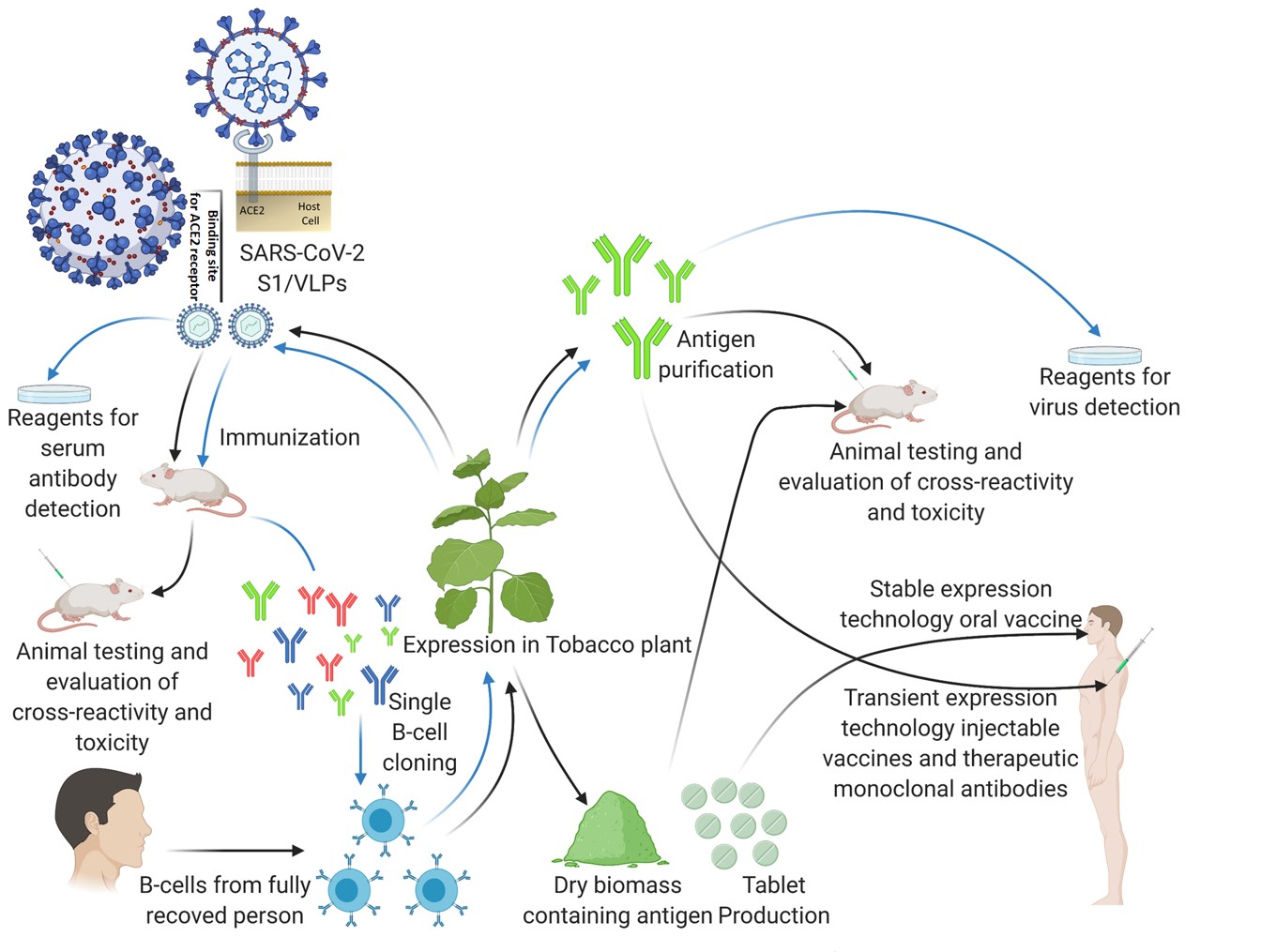 Fig.1 The utilization of plant-based expression systems in response to SARS-CoV-2 virus pandemicsfig.1,3
Fig.1 The utilization of plant-based expression systems in response to SARS-CoV-2 virus pandemicsfig.1,3
Creative Biolabs offers a rapid plant-based antibody production service utilizing Nicotiana benthamiana for transient expression, allowing for the initiation of protein expression in under three weeks from antibody sequence identification. This system provides a cost-effective alternative to traditional mammalian cell-based production by eliminating the need for complex bioreactors and reducing the risk of mammalian pathogen contamination. our plant expression platform is capable of producing a broad range of antibody formats, including full-size antibodies and various functional derivatives, offering a flexible and scalable solution for rapid antibody production. Throughout the whole process, we adhere to rigorous standards and employ advanced techniques to guarantee the integrity, purity, and functionality of the final antibody product. This commitment to quality ensures that our clients receive reliable and consistent results, thereby empowering their research and development endeavors.
The genetic sequence corresponding to the target antibody is inserted into a plant-compatible expression vector, which is subsequently transfected into Agrobacterium tumefaciens strains.
Step 2: Bacterial Culture PreparationRecombinant Agrobacterium strains harboring the antibody expression construct are cultivated under optimal growth conditions to achieve cell densities suitable for plant transformation.
Step 3: Plant TransformationA suspension of the engineered Agrobacterium is introduced into Nicotiana benthamiana specimens, either at the seedling or mature growth stage, using vacuum infiltration. This technique facilitates transient gene transfer into plant cell nuclei.
Step 4: Transient Protein SynthesisPost-infiltration, plants are maintained in controlled environmental chambers for 3-7 days to enable transient expression of the recombinant antibody within plant tissues.
Step 5: Protein ExtractionFollowing the incubation period, leaf tissue is homogenized, and total soluble proteins—including the synthesized antibody—are isolated through mechanical and chemical extraction methods.
Step 6: Protein Purification and CharacterizationThe crude extract undergoes affinity chromatography or related purification techniques to isolate the antibody. Yield and purity are quantified via enzyme-linked immunosorbent assay (ELISA) and electrophoretic analysis (e.g., SDS-PAGE).
Step 7: Product ValidationRigorous quality assurance protocols, including functional and biochemical assays, are implemented to confirm the antibody's structural integrity, specificity, and suitability for downstream applications.
Our plant-based antibody production system is highly versatile and can be adapted to various antibody formats, including:
Summary: Plant-based systems, particularly Nicotiana benthamiana, are being explored for the production of anti-HIV broadly neutralizing antibodies (bNAbs), due to their potential for cost-effectiveness and scalability.
Method: This study successfully expressed and characterized the targeted bNAb variants in glycoengineered N. benthamiana plants, and through in planta co-expression of tyrosyl protein sulfotransferase 1, introduced the crucial O-sulfated tyrosine modification in the antibody's CDR H3 loop.
Result: The resulting plant-produced bNAbs demonstrated similar structural and functional features, including potent neutralizing activity against subtype C viruses, compared to those produced in mammalian cells, highlighting the potential of plant-based systems for producing fully active bNAbs for HIV prevention and treatment.
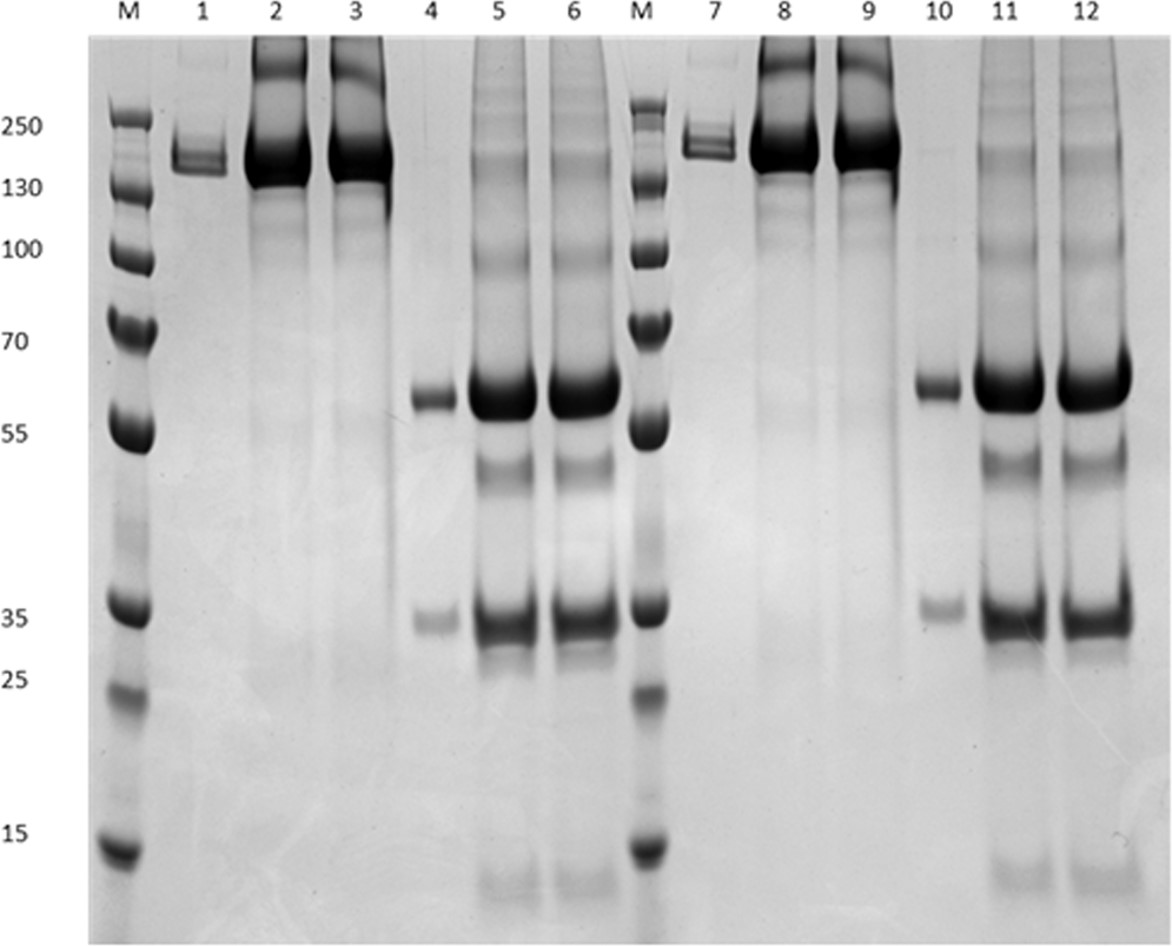 Fig.2 SDS-PAGE analysis.2,3 |
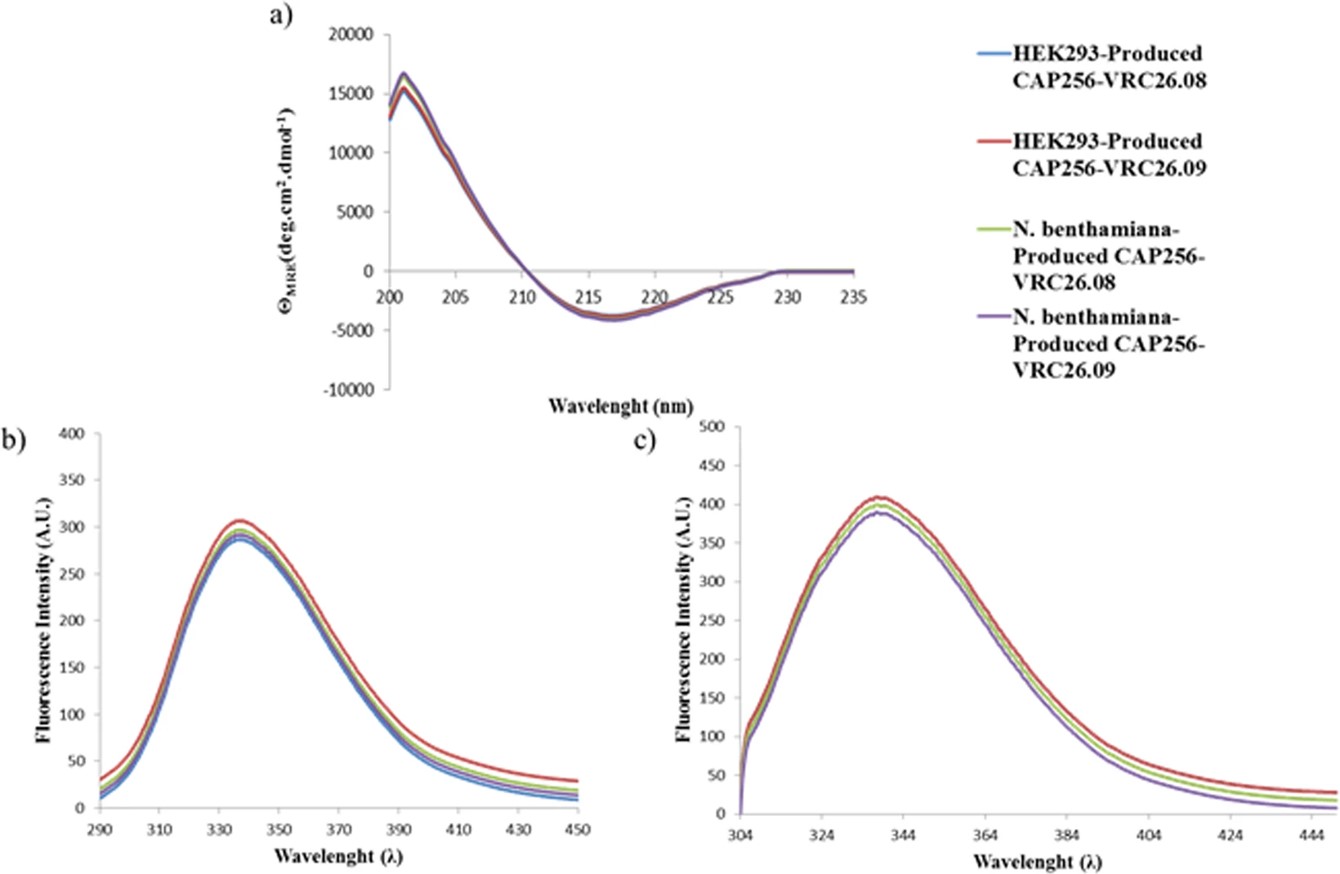 Fig.3 Structural analysis.2,3 |
Q1: What is the typical turnaround time for antibody production?
A1: The turnaround time ranges from 4 to 8 weeks, depending on the intricacy of the antibody and the amount needed. We emphasize fast delivery without sacrificing quality.
Q2: Is plant-based antibody production safe?
A2: Of course, with a far lower danger of human pathogen contamination, our plant-based methods provide a safe substitute for conventional mammalian cell culture. This makes the synthesis of therapeutic antibodies a consistent option.
Q3: Can you customize antibody glycosylation?
A3: Of course, our plant-based approach enables glycoengineering, thereby producing antibodies with specific glycosylation characteristics. Maximizing antibody performance and effectiveness depends on this in great part. Speak with us to investigate glycosylation engineering's possibilities for your antibodies.
At Creative Biolabs, our unwavering commitment is to empower your research and development with superior antibody production services. We achieve this by leveraging cutting-edge plant-based expression systems, ensuring the swift delivery of high-quality antibodies while maintaining cost-effectiveness. Our expertise and streamlined processes are dedicated to providing you with reliable and efficient solutions tailored to your specific antibody needs. If you are interested in using plant systems to quickly obtain high-quality antibody products, please contact us for further discussion.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION