Current CAR-T therapeutic approaches encounter persistent challenges, including suboptimal responses in solid malignancies and constrained durability of cellular persistence. To address these limitations, our next-generation CAR-T engineering platform employs molecular armoring techniques and microenvironmental reprogramming technologies, offering refined solutions to augment therapeutic efficacy and prolong functional activity in preclinical and clinical applications.
With unparalleled clinical success, CAR-T cell therapy has transformed hematologic tumor treatment. Many complicated biological hurdles stop this immunotherapeutic method from treating solid cancers. The immunosuppressive tumor microenvironment (TME) severely affects CAR-T cell invasion, cytotoxic activity, and in vivo persistence in solid malignancies. Solid tumors' intratumoral heterogeneity allows fractions of neoplastic cells to escape immune detection, promoting disease recurrence. We must develop next-generation CAR-T systems to address these limits. New technologies aim to resist immunosuppressive TME signals, optimize cellular trafficking to tumors, and increase antigen-recognition capacity to offset clonal variability. Such developments are necessary for solid tumor anticancer efficacy.
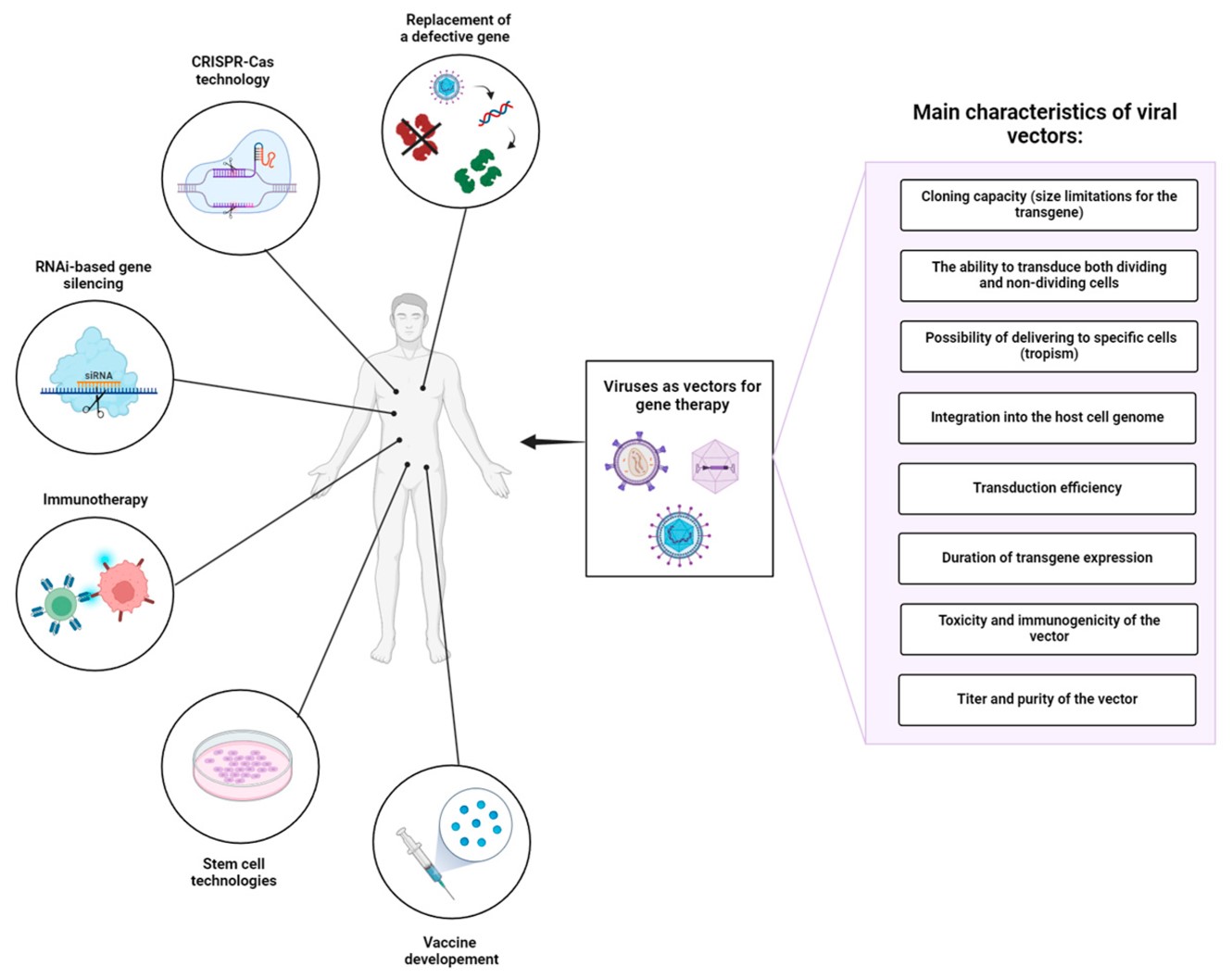 Fig.1 CAR-T therapy in solid tumors.1
Fig.1 CAR-T therapy in solid tumors.1
Creative Biolabs' next-generation CAR-T development service delivers end-to-end support for advancing CAR-T therapies targeting solid tumors. Our team crafts customized roadmaps to refine cell targeting capabilities, extend therapeutic persistence, and maximize clinical impact. Focusing on persistent hurdles in solid tumor treatment, we deploy a systematic methodology encompassing three critical dimensions: engineering next-generation CAR architectures with enhanced functionality, establishing streamlined manufacturing protocols, and deploying novel techniques to reshape immunosuppressive tumor microenvironments. By integrating these strategies, we enable researchers to bypass critical barriers in solid tumor immunotherapy and fast-track therapeutic candidates from concept to preclinical validation.
Our process initiates with comprehensive validation of the target antigen to confirm its specificity and capacity to trigger robust CAR-T cell activation. This involves confirming antigen specificity, quantifying expression patterns across healthy and diseased tissues, and systematically evaluating risks of off-target interactions. Validation data directly informs subsequent design parameters to balance efficacy with safety.
Following target validation and client requirements, we engineer chimeric antigen receptor (CAR) molecules tailored to therapeutic objectives. Our designs incorporate elements such as antigen-binding domains optimized for affinity/avidity, tunable hinge/spacer regions for spatial flexibility, and synergistic intracellular signaling domains (e.g., CD28 or 4-1BB costimulatory motifs paired with CD3ζ activation domains). Custom configurations, including dual-specificity CARs and cytokine-armored variants, are developed to address complex tumor microenvironments.
We employ clinically validated lentiviral or retroviral systems to deliver CAR constructs into primary T cells. Vector production protocols are rigorously standardized to achieve high-titer batches, with transduction parameters fine-tuned for cell type and CAR architecture. This ensures high-efficiency gene transfer while maintaining cellular fitness and consistent CAR surface expression.
We perform T cell activation, transduction, and expansion under optimized conditions to generate a sufficient number of functional CAR-T cells. This includes quality control assessments of CAR expression, cell viability, and phenotype.
A tiered testing strategy combines in vitro functional assays (real-time cytotoxicity, antigen-specific IFNγ/TNFα release) with in vivo PDX/CDX tumor models to validate therapeutic activity. Biodistribution studies and repeat-dose toxicity testing in immunocompetent models address safety parameters, including cytokine release syndrome (CRS) risk and on-target/off-tumor effects. All data packages are compiled to support IND-enabling studies.
Q1: Can your service help develop CAR-T cells that target specific solid tumor antigens?
A1: Our offerings help CAR-T cell treatments customized for certain solid tumor antigens to be developed. This covers enhanced CAR design processes to maximize targeting accuracy and full-cycle antigen validation. We advise setting up a visit to go over your particular antigen targets and project needs.
Q2: How does your service improve the persistence of CAR-T cells in the tumor microenvironment?
A2: Our method combines many engineering techniques to improve T cell durability: structural CAR alterations and cytokine-armored configurations address immunosuppressive microenvironments through processes our team can outline in technical talks.
Q3: What preclinical models do you use to evaluate CAR-T cell efficacy and safety?
A3: Combining 3D tumor models, patient-derived xenografts, and immune-competent murine platforms, our preclinical testing uses structured validation systems before clinical translation. This multi-modal method allows thorough evaluation of therapeutic activity and toxicological profiles.
Q4: Can you customize CAR designs to meet specific research needs?
A4: Yes, we specialize in custom CAR architectures optimized for unique research objectives. Modifications span extracellular binding domains, transmembrane regions, and intracellular signaling components. Our engineering team collaborates closely with clients to implement tailored solutions.
Creative Biolabs is a leading provider of CAR-T cell therapy development services, with a proven track record of success. This success is grounded in a combination of factors, including extensive experience in the field, a commitment to rigorous scientific methodology, and a focus on delivering tangible results for our clients. Our expertise spans the entire CAR-T cell therapy development pipeline, from initial target identification and CAR design to preclinical testing and clinical trial support. We have consistently achieved significant milestones in collaboration with our clients, contributing to the advancement of CAR-T cell therapies for various cancer indications. If you are suffering from the limited efficacy of your CAR-T therapy in solid tumors, contact us to accelerate your research.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION