Are you currently facing long development cycles for cell therapies, difficulty in achieving specific tumor recognition, or challenges in NK cell persistence and efficacy? Creative Biolabs' TCR-NK cell development service helps you accelerate the development of highly effective, tumor-specific cell therapies and enhance NK cell tumor recognition and persistence through advanced gene engineering, high-throughput screening, and innovative NK cell expansion and modification techniques.
The evolving cancer immunotherapy landscape faces challenges in precise tumor targeting and overcoming immunosuppression. CAR-T cell therapies, though successful in hematological malignancies, have limited efficacy and toxicities in solid tumors. Natural Killer (NK) cells offer a promising, safer alternative with inherent anti-tumor activity and lower risk of cytokine release syndrome and GvHD. However, native NK cells often lack sufficient specificity and persistence for effective solid tumor eradication. TCR-engineered NK (TCR-NK) cells address this critical unmet need by combining NK cell anti-tumor capabilities with the exquisite antigen specificity of T-cell receptors (TCRs). This engineering allows NK cells to recognize MHC-restricted tumor antigens, expanding their targeting repertoire and enhancing precision for safer, more effective cancer immunotherapies.
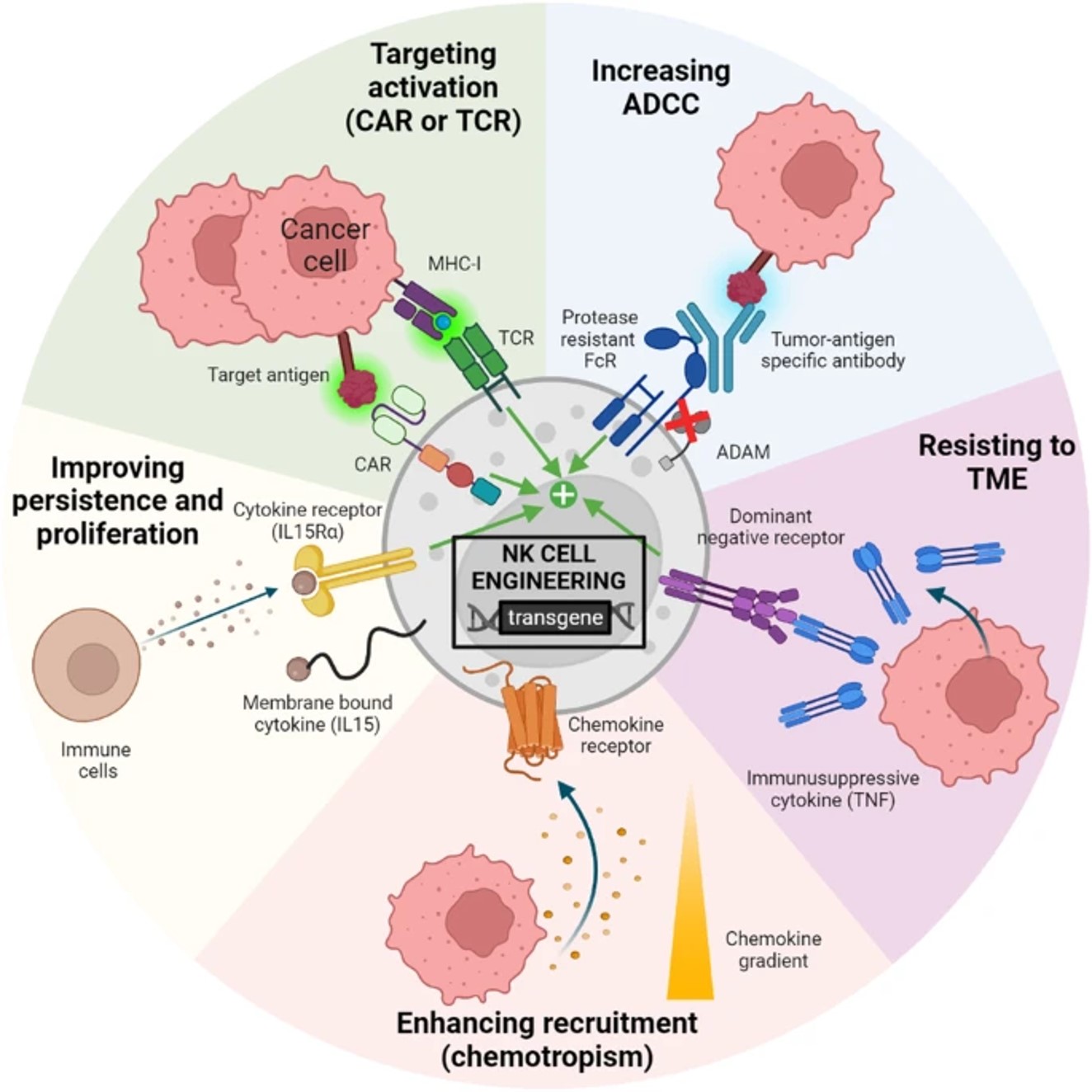 Fig.1 Various NK cell engineering strategies for enhanced functions.1,3
Fig.1 Various NK cell engineering strategies for enhanced functions.1,3
Creative Biolabs offers a comprehensive TCR-NK cell development service designed to empower your cancer immunotherapy research and development. Our service encompasses a streamlined workflow, from NK cell isolation and TCR gene transfer to functional validation and large-scale production, providing characterized cell lines and detailed data reports, typically within 12-20 weeks, all to enhance the precision and efficacy of your cell therapy projects with scientifically grounded solutions.
We begin by isolating NK cells from your provided source material. These cells are then expanded using optimized protocols to ensure a sufficient number of high-purity NK cells for subsequent engineering. This step ensures a robust starting population for modification.
Utilizing advanced viral or non-viral gene transfer methods, we introduce the desired TCR alpha and beta chain genes into the expanded NK cells. Our expertise ensures efficient and stable integration of the TCR genes into the NK cell genome.
Following gene transfer, we meticulously validate the expression of the introduced TCRs on the surface of the NK cells using techniques such as flow cytometry. We also assess the viability and purity of the engineered cells. This step confirms successful engineering.
The engineered TCR-NK cells undergo rigorous in vitro functional assays. This includes cytotoxicity assays (e.g., co-culture with target tumor cells to measure killing efficiency), cytokine release assays, and proliferation studies. We optimize conditions to ensure maximal tumor recognition and effector function.
For projects requiring larger quantities, we scale up the production of the optimized TCR-NK cells under stringent quality control measures. Comprehensive testing is performed to ensure product consistency, sterility, and potency.
This study successfully demonstrated the efficacy of T-cell receptor (TCR) expression in NK cells. This TCR complex, when properly assembled, led to robust degranulation, IFNγ production, and target cell killing. The findings highlight the therapeutic potential of TCR-engineered NK cells.
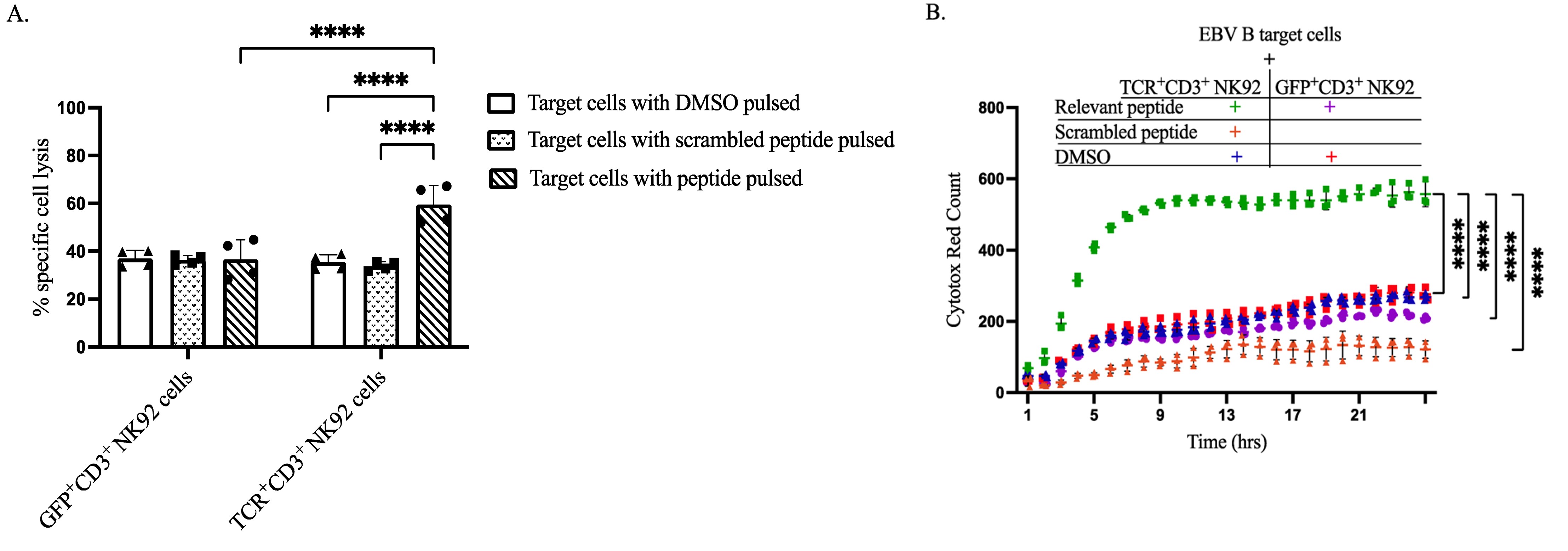 Fig.2 The cytotoxic activity of engineered NK cell subsets against target cells.2,4
Fig.2 The cytotoxic activity of engineered NK cell subsets against target cells.2,4
Q1: How does TCR-NK compare to CAR-NK or CAR-T cell therapies?
A1: TCR-NK cells offer distinct advantages, particularly in targeting intracellular antigens presented by MHC molecules, which CAR-based therapies cannot. While CAR-T cells have shown efficacy, TCR-NK cells leverage the innate safety profile of NK cells, potentially reducing risks like severe cytokine release syndrome and graft-versus-host disease, and offer potential for allogeneic "off-the-shelf" applications.
Q2: What is the typical turnaround time for a TCR-NK development project?
A2: The typical timeframe for our TCR-NK Cell Development Service ranges from 12 to 20 weeks. This duration can vary depending on the complexity of the target antigen, the specific TCR sequences involved, and the scale of engineered cell production required. We provide a detailed project timeline after initial consultation.
Q3: Can Creative Biolabs assist with regulatory aspects for clinical translation?
A3: While our primary service focuses on the development and characterization of TCR-NK cells, our team possesses extensive experience in preclinical development. We can provide data packages and guidance that support your regulatory submissions, and we are happy to connect you with our network of regulatory experts for comprehensive support.
To further support your immunotherapy research and development, Creative Biolabs also offers a suite of established services to help engineer your NK cells for improved functions:
Creative Biolabs is your trusted partner in advancing cutting-edge cell therapies. Our TCR-NK Cell Development Service provides a robust, efficient, and highly specific solution for enhancing tumor recognition and accelerating your therapeutic breakthroughs. Leveraging our deep scientific expertise and state-of-the-art platforms, we are dedicated to delivering high-quality, functionally validated TCR-NK cells tailored to your unique project needs. If you want to know more for our TCR-NK development service, please contact us with your meaningful projects.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION