Infection Management Solutions
Online Inquiry
Background Service Workflow Service Packages Advantages FAQs Why Choose Us
Next-generation CAR T-cell development presents complex infection challenges, including post-treatment infections, antimicrobial resistance, complex patient populations, and prolonged recovery times in biopharmaceutical projects. Creative Biolabs' infection management solutions mitigate these infection risks, enhance patient safety, and optimize therapeutic efficacy through proactive surveillance, rapid diagnostics, and targeted intervention strategies.
Why We Need to Develop Infection Management Solutions
The landscape of modern medicine, particularly in advanced therapeutics like gene and cell therapies, such as CAR T-cell therapy, has introduced unprecedented challenges in patient care. While these therapies offer revolutionary potential, they often induce severe immunosuppression and significant side effects, which dramatically increase patient susceptibility to a wide spectrum of infections. As highlighted in recent research concerning CAR T-cell therapy, managing these infections is critical for patient survival and overall therapeutic success. The ongoing threat of antimicrobial resistance further complicates treatment, underscoring the urgent necessity for comprehensive, proactive, and precise infection management solutions to improve patient outcomes and ensure the full benefit of innovative treatments.
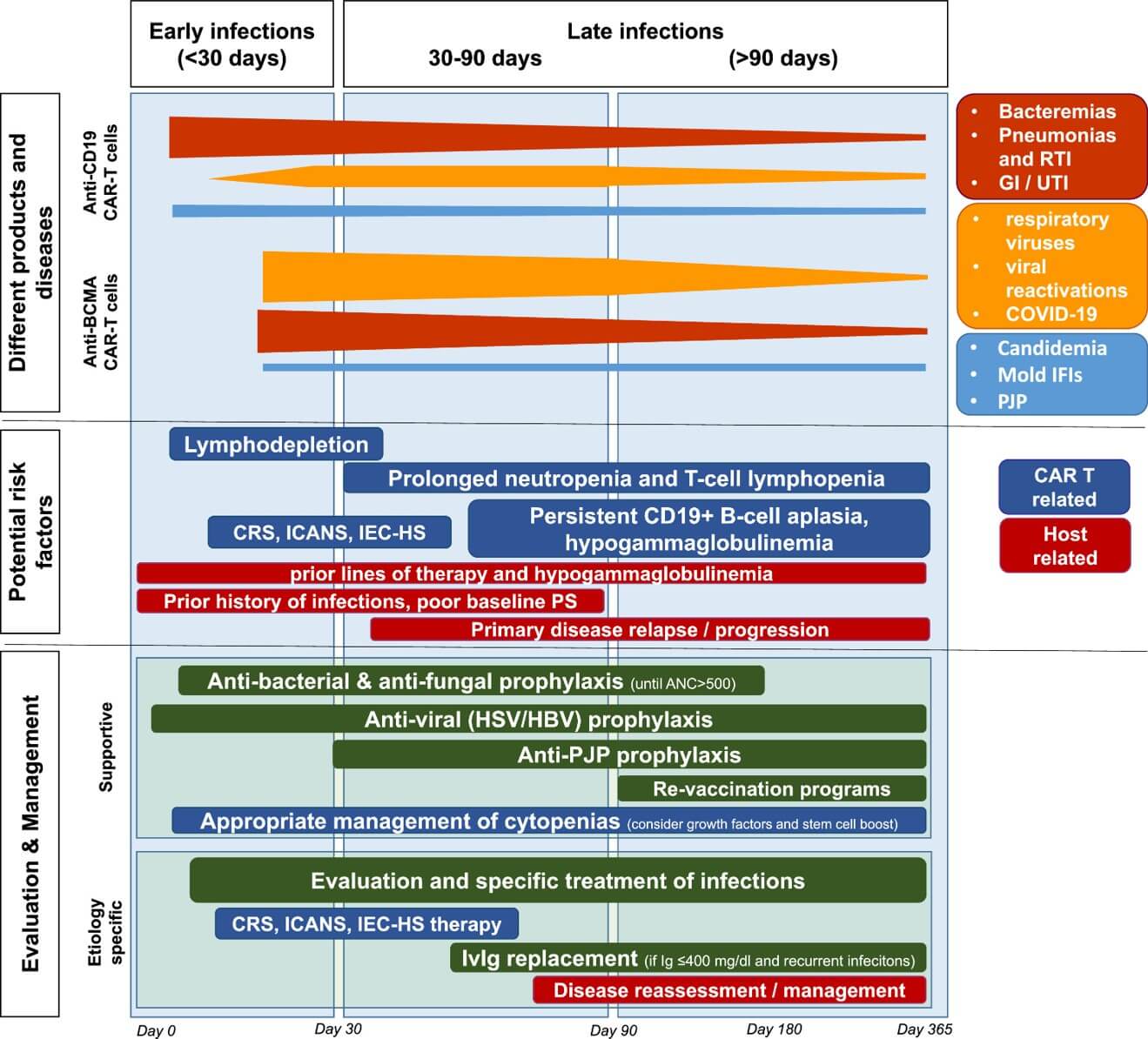 Fig.1 Infections involve the occurrence, underlying causes (etiologies), and their subsequent treatment (management).1
Fig.1 Infections involve the occurrence, underlying causes (etiologies), and their subsequent treatment (management).1
Infection Management Solutions at Creative Biolabs
Creative Biolabs offers a range of flexible service packages designed to address diverse infection management needs across various biopharmaceutical projects. Our solutions are tailored to combat infections originating from a multitude of sources, ensuring comprehensive protection and effective intervention. Our flexible packages can be customized and combined to create a bespoke infection management strategy that perfectly aligns with your project's unique requirements and objectives, providing robust solutions against various sources of infection.
Our Workflow
Step 1: Risk Assessment & Profiling: We analyze patient populations and therapeutic regimens to identify potential infection risks, creating a comprehensive risk profile.
Step 2: Pathogen Identification & Characterization: Advanced molecular and microbiological techniques are used to rapidly identify and characterize causative pathogens.
Step 3: Antimicrobial Susceptibility Testing (AST): We perform AST on identified pathogens to guide the selection of effective treatment options.
Step 4: Personalized Prophylaxis & Treatment Strategy Development: Tailored prophylaxis and treatment strategies are developed based on risk assessment, pathogen identification, and AST results.
Step 5: Monitoring & Follow-up: Continuous monitoring of patient status and treatment response ensures optimal outcomes and allows for adaptive plan adjustments.
Our Service Packages
This service provides robust platforms for the detection, identification, and characterization of bacterial pathogens. We offer a variety of assays including bacterial culture, molecular diagnostics (PCR, qPCR), and advanced sequencing for strain typing and resistance gene detection. This is crucial for precise diagnosis and guiding effective antibiotic therapies.
Focusing on respiratory viruses, this package includes rapid diagnostic assays for common and emerging viral pathogens (e.g., influenza, respiratory syncytial virus, SARS-CoV-2). Our services cover viral load quantification, genotyping, and antiviral susceptibility testing, supporting timely intervention and outbreak management.
Designed for monitoring and managing HSV infections, particularly in immunocompromised patients where reactivation is a significant concern. This service provides sensitive detection of HSV DNA/RNA, viral load monitoring, and assessment of antiviral drug efficacy against reactivated virus, enabling proactive management.
Similar to our HSV service, this package focuses on VZV reactivation, a common complication in patients with compromised immune systems. We offer precise detection of VZV, viral load monitoring, and evaluation of antiviral responses to mitigate the risks of shingles and other VZV-related complications.
Addressing the increasing prevalence of fungal infections, especially in vulnerable patient populations, this service provides comprehensive diagnostic assays for various fungal pathogens. This includes culture-based methods, molecular detection, and antifungal susceptibility testing to guide appropriate and effective antifungal treatment.
Our Service Advantages
-
Rapid pathogen identification
-
Timely and targeted interventions
-
Reduced infection rates
-
Optimized patient recovery
-
Minimizing treatment complications
-
Enhanced therapeutic outcomes
-
Streamlined project timelines
-
Reducing overall healthcare costs
FAQs
Q1: What types of infections can Creative Biolabs' solutions help manage?
A1: Our solutions cover a broad spectrum of infections, including bacterial, viral, and fungal pathogens, particularly those prevalent in immunocompromised patient populations undergoing advanced therapies like CAR T-cell therapy.
Q2: How quickly can Creative Biolabs identify pathogens and provide results?
A2: We prioritize rapid diagnostics. While exact timelines depend on the complexity of the sample and pathogen, our advanced molecular techniques are designed for speed, often providing critical identification and susceptibility results within days, enabling timely clinical decisions.
Q3: How do Creative Biolabs' solutions compare to standard infection control practices?
A3: Our solutions go beyond standard practices by offering a proactive, highly personalized, and technologically advanced approach. We integrate comprehensive risk assessment, rapid molecular diagnostics, and tailored intervention strategies, providing a more precise and effective shield against infections than conventional methods.
Q4: What support does Creative Biolabs provide after the initial strategy implementation?
A4: Our commitment extends beyond initial implementation. We offer continuous monitoring, ongoing consultation, and adaptive adjustments to the infection management plan based on real-time data and evolving clinical needs, ensuring sustained patient safety and project success. We encourage you to reach out for a detailed discussion.
Related Services
To further support your research and development goals, Creative Biolabs offers a suite of complementary services that can be integrated with our infection management solutions:
Why Choose Us
Creative Biolabs is your dedicated partner in navigating the complexities of infection management within advanced biopharmaceutical development. Our comprehensive Infection Management Solutions, backed by deep scientific expertise and cutting-edge technologies, are designed to safeguard patient health, minimize risks, and accelerate your therapeutic outcomes. Please don't hesitate to contact us for more details.
Reference
-
Shahid, Zainab et al. "Best Practice Considerations by The American Society of Transplant and Cellular Therapy: Infection Prevention and Management After Chimeric Antigen Receptor T Cell Therapy for Hematological Malignancies." Transplantation and cellular therapy vol. 30,10 (2024): 955-969. doi:10.1016/j.jtct.2024.07.018. Distributed under Open Access License CC BY 4.0, without modification.






 Fig.1 Infections involve the occurrence, underlying causes (etiologies), and their subsequent treatment (management).1
Fig.1 Infections involve the occurrence, underlying causes (etiologies), and their subsequent treatment (management).1