cGMP lentivirus production for CAR-T therapies is a critical step in generating safe and effective viral vectors for gene modification. This process follows stringent guidelines to ensure that the viral vectors used for CAR-T therapies meet regulatory standards for quality, safety, and efficacy. Creative Biolabs provides high-quality, clinical-grade lentiviral vectors tailored to meet the needs of cutting-edge gene therapies.
cGMP lentivirus is crucial in advanced medical applications, particularly for CAR-T therapy, gene therapy, and immunotherapy. In CAR-T therapy, lentiviral vectors are used to deliver chimeric antigen receptor (CAR) genes into T cells, enabling them to specifically target and destroy cancer cells. Lentivirus is also widely used in gene therapy to treat genetic disorders by delivering corrective genes to patient cells, ensuring safe and effective therapeutic outcomes. Additionally, lentiviral vectors are applied in stem cell modification, vaccine development, and immunotherapies to engineer cells that fight diseases such as cancer and viral infections. cGMP-compliant production guarantees that these vectors meet the rigorous safety, potency, and purity standards required for clinical and commercial use.
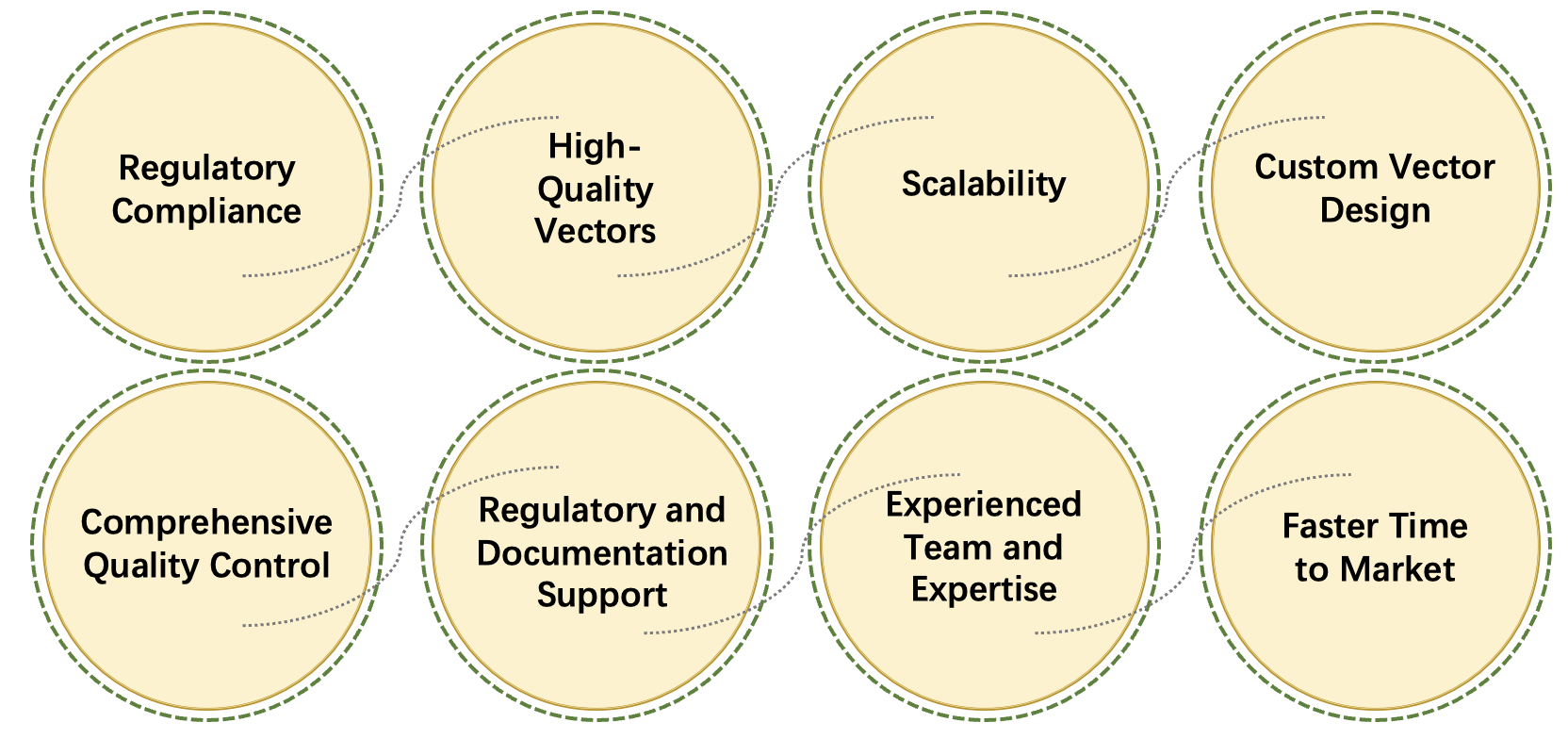
Our cGMP Lentivirus Production service for CAR-T therapy provides high-quality, clinical-grade lentiviral vectors tailored to meet the needs of cutting-edge gene therapies. Adhering to stringent regulatory standards, we offer scalable production platforms, from research-grade to commercial-scale manufacturing, ensuring safety, efficacy, and regulatory compliance. With advanced vector design, producer cell line development, and purification processes, we guarantee superior vector yield and purity. Comprehensive quality control, including sterility testing and potency validation, ensures your vectors are ready for clinical trials or therapeutic use, backed by full regulatory support.
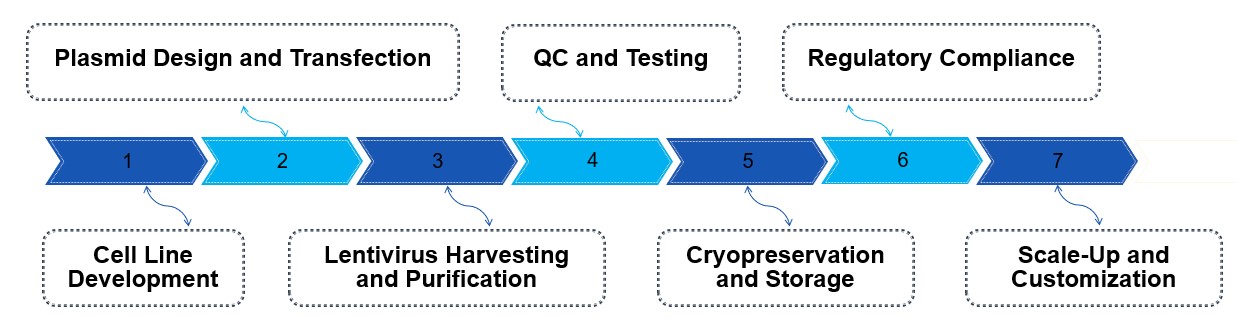
Our CGMP lentivirus production process for CAR-T involves several key steps to ensure quality and safety. First, producer cells (typically HEK293T) are developed and optimized for high-yield lentiviral vector production. Plasmids containing the CAR gene and necessary helper sequences are transfected into these cells. Once the virus is produced, it is harvested and purified using techniques like tangential flow filtration (TFF) and ultracentrifugation to remove impurities. Quality control testing ensures sterility, potency, and the absence of replication-competent lentivirus. Finally, the purified lentiviral vectors are cryopreserved, stored under stringent conditions, and fully documented to meet regulatory standards.
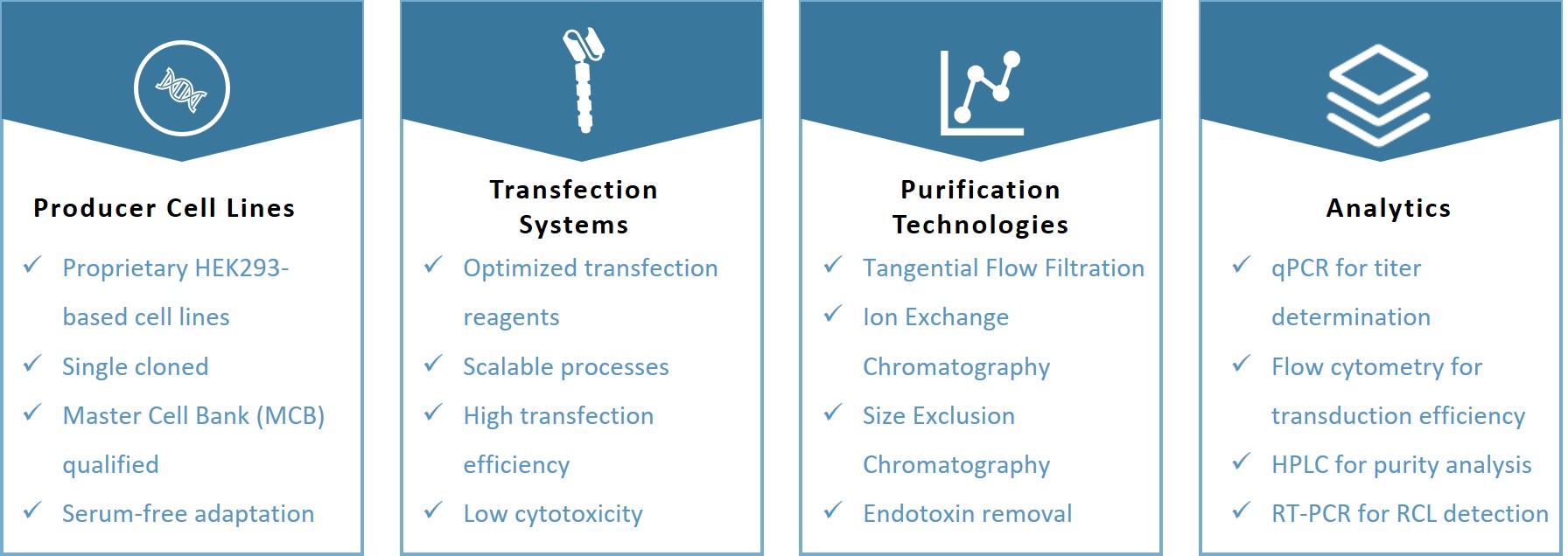
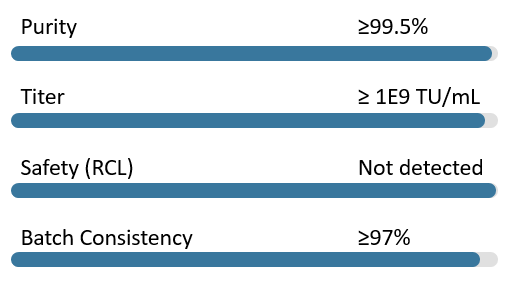
Our rigorous quality control processes ensure the safety, efficacy, and consistency of your CAR-T lentiviral vectors.
QC Testing Panel
| Identity | Restriction Enzyme Mapping |
|---|---|
| Restriction Enzyme Mapping | UV spectrophotometry at 260 nm |
| Concentration | UV spectrophotometry |
| UV spectrophotometry at 260 nm | pH meter |
| Purity (OD260/OD280) | qPCR |
| UV spectrophotometry | Agarose gel electrophoresis |
| pH | USP <71> sterility test |
| pH meter | LAL assay |
| Titer | qPCR/ Marker Rescue Assay |
| qPCR / TCID50 | ELISA |
Quality Metrics
cGMP Compliance
Our manufacturing facilities are designed and operated in compliance with current cGMP guidelines, ensuring the highest standards of quality.
All manufacturing processes are fully validated to ensure consistency, reliability, and scalability across batches.
Robust quality management systems in place, including deviation management and change control.
Comprehensive documentation system ensuring full traceability and compliance with regulatory requirements.
How do you ensure high purity and low endotoxin levels?
Our closed-system purification platform uses tangential flow filtration and chromatography to remove impurities (e.g., host cell DNA/proteins) and maintain low endotoxin levels, ensuring a safer product for cell transduction.
Can you handle complex custom CAR-T vector production?
Yes. We specialize in custom production, optimizing the process for complex CAR designs. We analyze your CAR cassette, select the ideal producer cell line, and tailor purification to maximize yield and integrity.
How does your scalability compare to in-house or academic facilities?
We offer seamless, cGMP-compliant scalability from clinical to commercial volumes without the need for capital investment or facility validation. The de-risk production accelerates timelines, allowing you to focus on clinical development.
Leveraging Creative Biolabs' cGMP CAR-T lentivirus production service in our research has notably enhanced batch-to-batch uniformity in the transduction efficiency of our T cells. The report on replication-competent lentivirus testing provided by the team was key to meeting our internal QA standards - a critical issue that had been a significant challenge with our previous vendor. Dr. A**be.
The vector produced by Creative Biolabs delivered a significantly higher functional titer than our previous pre-cGMP process, which translated to superior T-cell transduction rates (up to 85%) in our target cell population. The Creative Biolabs team provided exceptional guidance on cryopreservation stability, a crucial detail often overlooked by other CDMOs. Pl G
To receive detailed technical specifications, regulatory documentation examples, or a custom project quote, please reach out to our expert team.
Looking to advance your CAR-T therapy development with our cGMP lentivirus production services? Contact us today to explore how we can meet your specific project needs and support both your research goals. Our dedicated team of experts is ready to provide answers to your questions and offer customized solutions that will help fast-track your progress from research to therapeutic application.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION