Next-Gen CAR-T Toxicity Management Solutions
Online Inquiry
Introduction Strategies Service Portfolio Workflow Required Materials Highlights Customer Reviews FAQs Extended Services
Industry-wide Need for CAR-T Toxicity Management Solutions
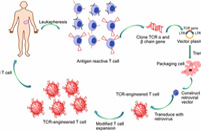
CAR-T therapies have revolutionized hematological cancer treatment but remain challenged by serious toxicities such as CRS and ICANS. There is an urgent industry-wide need for advanced mitigation strategies to ensure therapeutic safety and scalability. Are you facing high cytokine release, neurotoxicity risks, or unpredictable infections in CAR-T programs? Next-gen CAR-T toxicity management solutions at Creative Biolabs help mitigate safety liabilities, ensure translational viability, and improve therapeutic precision through targeted immunotoxicity assays, predictive biomarkers, and modular CRS/ICANS intervention systems.
Creative Biolabs directly addresses the core challenge of uncontrolled immunotoxicity in CAR-T therapy. Our modular platform enables drug developers to proactively assess and prevent treatment-induced cytokine storms, neuroinflammation, and systemic immunopathology using validated preclinical models and real-time toxicity scoring systems. Clients benefit from personalized design recommendations, and robust datasets.
Discover How We Can Help – Request a Consultation
Strategies
Multi-Phase Monitoring
From preclinical modeling to patient stratification, we implement dynamic toxicity thresholds tailored to antigen density and tumor burden.
Biomarker-Based Prediction
Integrating IL-6, IFN-γ, and GM-CSF cytokine kinetics with machine-learning-driven toxicity risk scores.
In Situ Immune Control
Design of safety switches (e.g., suicide genes, ON/OFF toggles) embedded in CAR constructs to abort therapy in severe reactions.
Multi-Target Engineering
Utilizing tandem CARs to lower off-tumor effects and improve antigen specificity.
Immune Checkpoint Tuning
Integrating PD-1/CD28 switch receptors to balance efficacy and immune restraint.
Our Toxicity Management Service Portfolio
Comprehensive design and testing of CAR-T constructs with IL-6 surge mitigation, cytokine release assays, and real-time monitoring. Includes preclinical modeling, cytokine sink development, and comparative CRS scoring.
Specialized solutions for neurotoxicity risk evaluation using BBB penetration assays, neuroinflammatory biomarker panels, and co-culture systems to prevent or reduce ICANS in CAR-T development and application.
Proactive infection surveillance post-CAR-T therapy including latent virus reactivation screening, microbiota analysis, and immunosuppression-linked risk assessments to prevent complications and enhance patient safety.
Detection and suppression of HLH-like symptoms from overactivated CAR-T or NK cells. We offer IFN-γ and ferritin tracking, NK hyperactivation assays, and predictive early-warning models.
Assessment of CAR-induced thrombocytopenia and bone marrow suppression. Includes flow cytometric lineage tracking, megakaryocyte survival assays, and hematologic adverse event forecasting.
Targeted evaluation and control of CAR-T–related coagulation disorders. Services include D-dimer and fibrinogen monitoring, platelet function testing, thrombin generation assays, and risk modeling to enable timely intervention and improve patient outcomes.
Integrated assessment of tumor inflammation-associated neurotoxicity combining regional cytokine mapping, transcriptomic profiling, and in vivo behavior analysis to uncover localized risks in sensitive tissues.
Advanced tissue cross-reactivity screening using IHC, flow cytometry, and antigen density analysis to identify unintended off-tumor binding and reduce systemic toxicity risks.
Workflow
1. Initial Consultation & Safety Design Planning
We begin with a detailed consultation to understand your CAR structure, antigen targets, intended indication, and prior toxicity concerns. This helps tailor a custom toxicity risk framework. We discuss design needs like switchable CARs, dual-target logic gates, or suicide gene integration strategies.
2. Risk Prediction Modeling & Assay Selection
We implement AI-enhanced immune profiling tools and published biomarker risk scores to simulate patient-specific toxicology scenarios. Based on this, we recommend optimal in vitro models (e.g., PBMC co-culture, cytokine multiplex assays) and in vivo systems for immune and neurotoxicity simulations.
3. Toxicity Control Engineering & Platform Setup
Our team assists in the design and generation of CAR constructs embedded with desired control modules such as iCasp9, SynNotch kill-switches, or cytokine traps. Models are validated in flow cytometry, ELISA-based cytokine release, BBB permeability, and neuroinflammatory screens.
4. Data Collection, Quantitative Evaluation & Alert Threshold Mapping
Data from preclinical testing are rigorously analyzed against control cohorts. We generate precise toxicity index reports with actionable metrics, alert thresholds for IL-6, GM-CSF, IFN-γ, and identify off-target reactivity zones across tissue panels.
5. Final Report, Optimization Feedback
Clients receive a full technical report with recommendations on construct redesign, switch tuning, or dosage adjustments.
Required Starting Materials
-
Complete CAR construct map (scFv, spacer, transmembrane, signaling domains)
-
Tumor expression and healthy tissue expression data for target antigen
-
Anticipated cytokine panel for safety profiling or thresholds (e.g., IL-6, TNF-α)
Highlights
Full-Spectrum Safety Coverage
We cover all major CAR-T adverse events—CRS, ICANS, coagulopathy, TIAN, off-tumor effects—with distinct modules for each, ensuring clients get holistic and modular solutions.
Mechanism-Driven Intervention Tools
Our approaches are rooted in immune biology—targeting key molecules like IL-6, IFN-γ, and GM-CSF with validated inhibitors and decoy receptors—providing mechanism-guided design support.
Flexible Integration with Any Construct
Whether you use lentivirus, transposon, or mRNA systems, our solutions adapt. We offer seamless integration into standard, tandem, or inhibitory CAR platforms.
Rapid Turnaround & Expert Collaboration: Our team of immunologists and regulatory experts provides responsive support and design revisions throughout your program—ensuring reduced downtime and increased regulatory confidence.
Experience the Creative Biolabs Advantage – Get a Quote Today
Customer Reviews
[Early CRS Detection] Using Creative Biolabs' CAR-T toxicity platform significantly improved our CRS prediction during preclinical testing in CD19 models. We were able to fine-tune IL-6 response thresholds and avoid costly delays. Mar 2025, Dr. J** Lin*
[Safe Clinical Entry] Incorporating suicide switches designed by Creative Biolabs gave our regulatory team the confidence to move our dual-target CAR-T into Phase I trials. No dose-limiting toxicities reported. Jan 2025, Dr. B** Yang*
[Reduced Neurotoxicity] With their ICANS screening tools, we identified unintended BBB-penetrating constructs early and optimized linker design to mitigate risk. Their team was instrumental in getting approval for our IND package. Apr 2025, Dr. M** Ruiz*
FAQs
Can these toxicity management solutions be adapted to solid tumor CAR-T therapies?
Yes. We customize CRS and ICANS controls to address the microenvironment complexity and antigen heterogeneity of solid tumors.
How do your suicide switch systems work in practice?
Our systems use inducible gene modules (e.g., iCasp9) triggered by small molecules to safely terminate CAR-T activity upon toxicity signs.
Can I integrate toxicity solutions with off-the-shelf CAR-T constructs?
Yes. We offer retrofitting services to embed safety modules into existing lentiviral or mRNA-based CAR platforms.
How early should I begin incorporating toxicity mitigation?
Ideally, during preclinical development or even construct design. Early inclusion reduces downstream risks and improves clinical success.
Extended Services
From Creative Biolabs' CAR-T Solutions, clients may also benefit from these integrated services to streamline development and reduce risks:
We provide tailored CAR designs, including tandem and inhibitory CARs, to enhance specificity and reduce off-target toxicity, with optimized signaling domains for function and persistence.
We offer cytotoxicity, cytokine release, and activation assays to verify CAR-T function and potency, supporting preclinical optimization and regulatory submissions.
Our PDX and humanized mouse models assess antitumor efficacy, persistence, and in vivo safety in translationally relevant settings.
We develop and validate cytokine and immune response biomarkers like IL-6 and GM-CSF for monitoring safety, efficacy, and patient stratification.
Creative Biolabs is your trusted partner in developing safe and effective CAR-T cell therapies. With cutting-edge tools and a proven track record in immunotoxicity management, we are ready to support your discovery and preclinical pipeline. Contact Our Team for More Information and to Discuss Your Project.