Creative Biolabs specializes in the development and manufacturing of CAR-T cells. Recognizing the pivotal role of RNA in the realm of gene therapy and immunotherapy, we offer comprehensive in vitro transcription (IVT) RNA manufacturing services for CAR-T, enabling enhanced therapeutic efficacy and safety. Our expertly tailored services ensure the highest quality and efficacy, empowering our clients to pioneer groundbreaking therapeutic solutions.
GMP-like mRNA refers to mRNA that is produced following the guidelines and principles of GMP but may not fully comply with all GMP requirements. It is often used for research and preclinical purposes where full GMP compliance is not mandatory but high quality and safety standards must still be maintained. At Creative Biolabs, we offer GMP-like mRNA manufacturing tailored for CAR-T therapies. This service aligns with GMP standards, ensuring high quality and safety for mRNA products used in therapeutic applications.
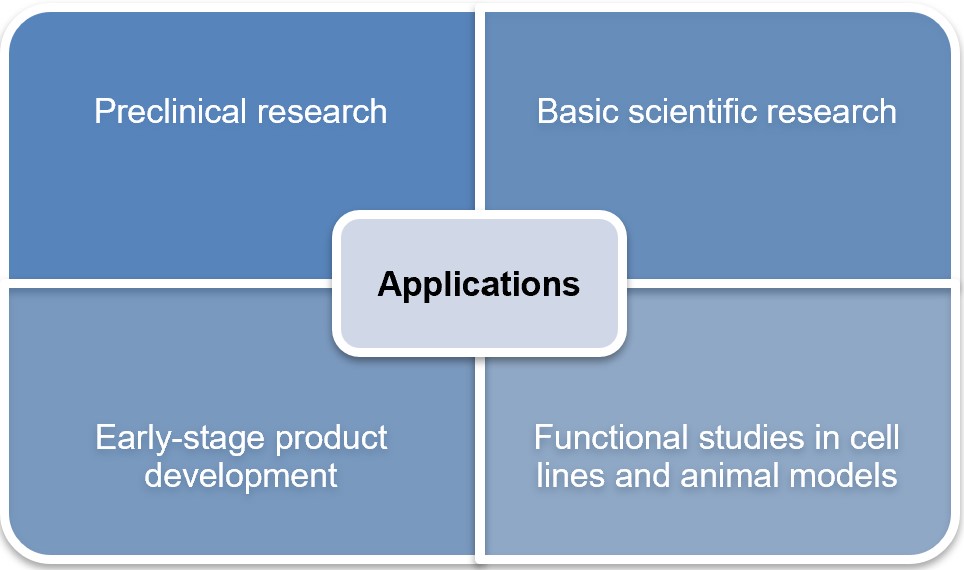
At Creative Biolabs, we have developed a state-of-the-art GMP-like mRNA manufacturing platform. Our platform integrates the latest advancements in mRNA synthesis, purification, and quality control to deliver mRNA products that meet stringent regulatory and clinical standards. By following GMP-like standards, we ensure every batch of mRNA is produced with consistency, purity, and stability, minimizing risks associated with batch-to-batch variabilities.
Our lab is equipped with world-class facilities dedicated to the manufacturing of mRNA and lipid nanoparticles (LNP) encapsulated formulations. Our facilities adhere to the highest standards of quality and compliance, ensuring that our production environments are contaminant-free and optimized for the highest efficiency.
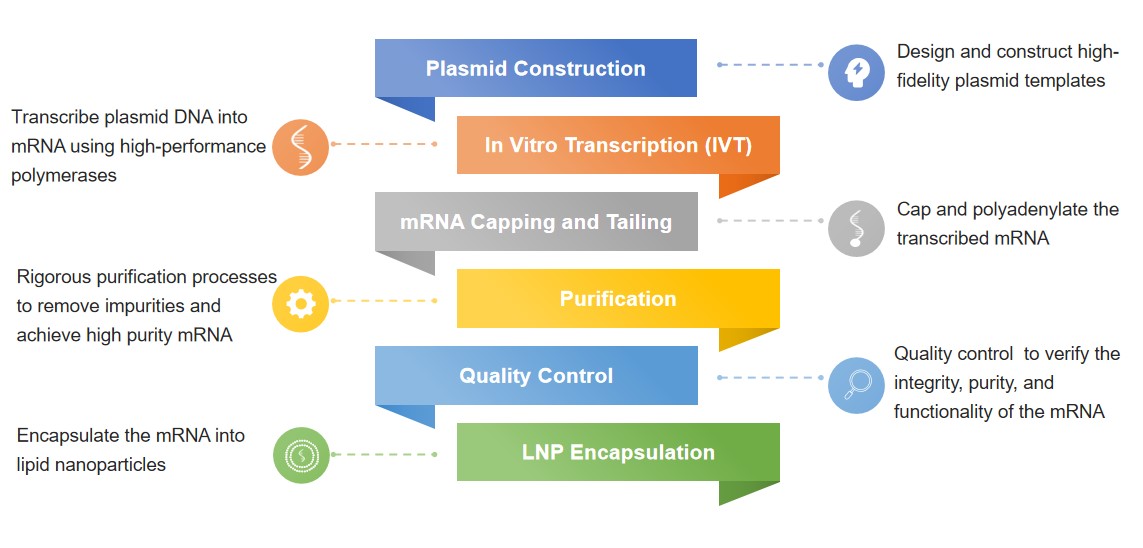
Our manufacturing process starts from plasmid DNA templates that are meticulously designed and validated. The IVT is carried out using T7 RNA polymerase-driven transcription systems, ensuring the synthesis of mRNA with high fidelity. Post-transcriptional modifications, including capping and polyadenylation, are performed to enhance the stability and translational efficiency of the mRNA. Each batch undergoes rigorous quality control testing, including purity assays, sequence verification, and functionality tests, all conforming to GMP-like standards. The platform is designed to be scalable, providing flexibility for various levels of production needs, whether for small-scale research or large-scale preclinical applications.
Creative Biolabs provides flexible, scalable GMP-like mRNA manufacturing designed to accelerate your CAR-T development programs.
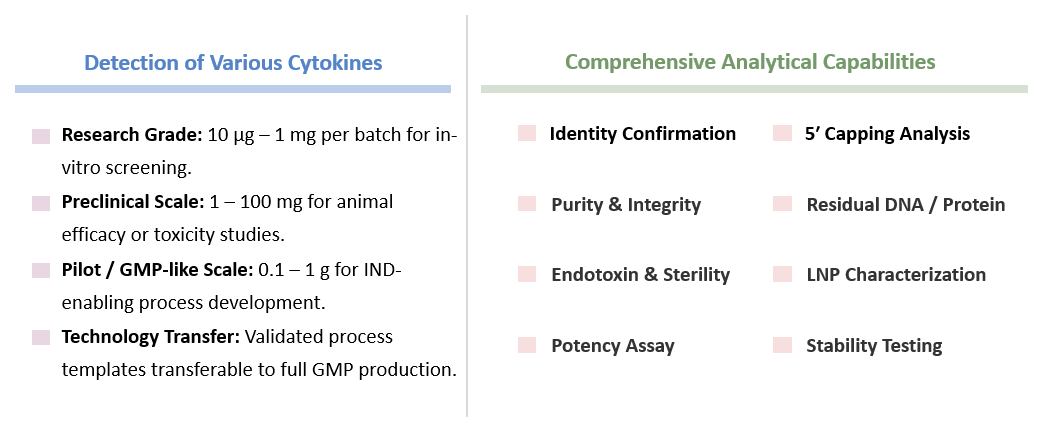
To guarantee the highest level of reproducibility, safety, and translational readiness, Creative Biolabs performs extensive quality control on every GMP-like CAR-T mRNA batch. All assays are conducted under GMP-like conditions with traceable documentation and qualified analytical instruments.
| Category | Typical Indicators | Analytical Methods |
|---|---|---|
| mRNA Purity & Integrity | ≥ 95 % full-length transcript; minimal truncated fragments | Agarose gel / Capillary electrophoresis |
| Residual Impurities | Residual DNA < 10 ng/mg; Protein < 100 ng/mg; Endotoxin < 0.1 EU/µg | qPCR, ELISA, LAL assay |
| 5' Capping Efficiency | ≥ 90 % capped transcripts | HPLC or LC-MS |
| Poly(A) Tail Length | ~120 ± 10 nt for optimized translation | Enzymatic digestion + gel analysis |
| Modified Base Ratio | > 95 % pseudouridine | LC-MS profiling |
| Fragment Size Distribution | ≥ 90 % full-length ≥ target nt length | Bioanalyzer |
| LNP Encapsulation Efficiency | > 85 % encapsulation | Dye assay |
| Particle Size & PDI | 80–120 nm average; PDI ≤ 0.2 | Dynamic Light Scattering |
| Zeta Potential | −5 to −15 mV | Zetasizer analysis |
| Product Stability | ≥ 90 % integrity after 3 months at −80 °C | HPLC / qPCR / DLS |
| Functional Potency | Verified in vitro translation and CAR expression | Flow cytometry / Western blot |
How is batch traceability ensured?
Each batch is assigned a unique internal tracking code and full batch record, covering raw materials, operators, equipment, and environmental logs. This enables complete traceability from the starting template to the final mRNA-LNP formulation.
Can the GMP-like mRNA be used in animal studies or IND-enabling experiments?
Yes. GMP-like materials are specifically designed for in vivo animal studies, preclinical evaluation, and IND-supporting toxicology tests. However, direct clinical use in humans requires GMP-grade manufacturing.
Do you provide formulation or LNP optimization support?
Yes. Our team offers custom lipid formulation and encapsulation optimization to improve RNA delivery, stability, and transfection performance for CAR-T and other cell therapy applications.
"Reliable partner for our preclinical CAR-T mRNA development." The Creative Biolabs team delivered consistent, high-purity mRNA batches with excellent technical documentation. The GMP-like workflow helped us validate our CAR construct efficiently. - B***D.
"Seamless transition to GMP manufacturing." We started with GMP-like batches for preclinical work and later upgraded to full GMP production without changing our validated process. The transfer was smooth and well-supported. – H***P.
At Creative Biolabs, we are committed to advancing the development of CAR-T therapies with our robust and reliable mRNA and LNP manufacturing services. Partner with us to unlock the potential of your therapeutic innovations and make strides towards transformative healthcare solutions. Please contact us for your GMP-like CAR mRNA project.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION