Creative Biolabs stands at the forefront of innovative cell and gene therapy solutions by providing global CDMO services for CAR-T therapy development. As part of our extensive suite of offerings, we specialize in in-vitro transcription (IVT) RNA services manufacturing to facilitate the production and scalability of CAR-T therapies. Our commitment to excellence and quality ensures that every stage of the CAR-T development process is meticulously managed to meet the unique needs of our clients.
At Creative Biolabs, our expertise extends across various domains, and we are particularly proud to offer advanced IVT RNA services tailored to the specialized needs of CAR-T cell therapy. We have developed a Good Manufacturing Practice (GMP) mRNA manufacturing platform that is designed to provide high-quality CAR-mRNA. Our platform ensures that the production of mRNA is precise, scalable, and free from impurities, which are crucial for maintaining the safety and efficacy of the resulting CAR-T cells. The platform is optimized for both small-scale and large-scale production, which caters to different phases of CAR-T therapy development—from preclinical studies to clinical trials and commercialization. This flexibility allows for the efficient scale-up of mRNA production, ensuring a seamless transition through the various stages of therapy development.
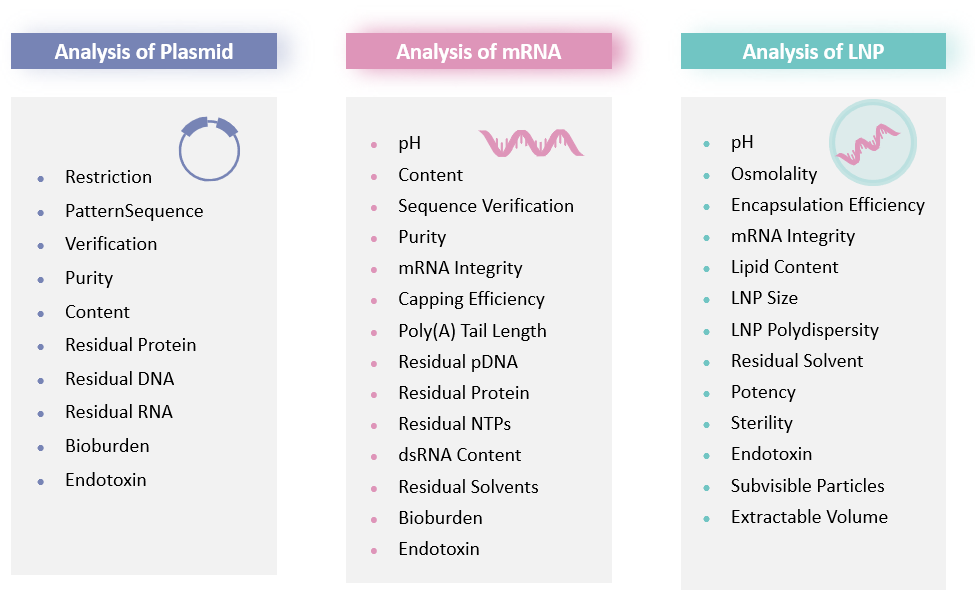
Our comprehensive suite of analytical methods for plasmid, mRNA, and mRNA-LNP products ensures final product quality. We support your project from in-process controls and release testing through to stability studies, all tailored to your specific requirements.
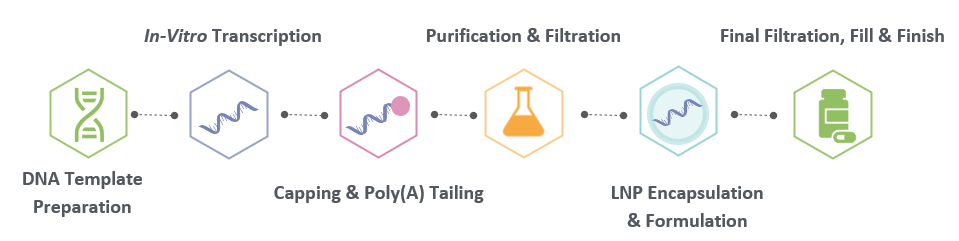
Our proprietary process combines precision engineering, traceable documentation, and automated control to deliver mRNA suitable for CAR-T applications and clinical translation.
| Attribute | QC Test |
| Purity, Concentration, and Integrity Analysis | UV-Vis spectroscopy, agarose gel electrophoresis, capillary gel electrophoresis |
| mRNA Sequence Analysis | Sanger sequencing or next-generation sequencing (NGS) |
| Residual DNA Analysis | qPCR |
| Endotoxin Testing | KCA assay |
| Microbial Testing | Sterility testing, bioburden testing |
| Protein Contamination | Western Blot, ELISA |
| Capping and Poly A tail Analysis | LC-MS, capillary gel electrophoresis |
Creative Biolabs offers a comprehensive suite of CAR-IVT RNA and LNP manufacturing services designed to accelerate the development of CAR-T cell therapies. Our advanced GMP mRNA manufacturing platform and state-of-the-art facility ensure that we consistently deliver high-quality, scalable solutions that meet the rigorous demands of modern therapeutic development. Partner with us to leverage our expertise and drive your CAR-T projects toward successful clinical and commercial outcomes.
Creative Biolabs offers customizable scales to meet each stage of CAR-T program development.
| Production Level | Typical Batch Yield | Intended Use |
|---|---|---|
| Research Grade | 10 µg – 1 mg | Feasibility & screening studies |
| Preclinical Scale | 1 – 100 mg | In-vivo / toxicity studies |
| GMP-like Scale | 0.1 – 1 g | IND-enabling production |
| Full GMP Transition | > 1 g | Clinical trials / regulatory submission |
What is the difference between GMP-like and full GMP manufacturing?
GMP-like follows the same controlled environment, qualified personnel, and analytical testing as full GMP, but is intended for preclinical or IND-enabling studies. Full GMP adds regulatory audits, QP release, and documentation for clinical use.
Can I provide my own plasmid template?
Yes. Clients may supply verified plasmids, and we will perform sequence validation and process optimization for IVT.
What are the typical deliverables?
You will receive mRNA material, full analytical reports, CoA, batch record, and storage recommendations.
Is the product suitable for preclinical or IND studies?
Absolutely. Our GMP-like mRNA meets preclinical/IND readiness standards and can be directly scaled to full GMP.
"Creative Biolabs delivered our CAR-T mRNA faster than expected, with exceptional purity and consistent quality. Their documentation made our IND package submission seamless." — B***C.
"We transitioned from GMP-like to full GMP with zero process changes. Highly recommend their technical team." — H**P.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION