Clinical CDMO services must adhere to stringent regulatory requirements set by authorities. This encompasses thorough documentation and quality assurance procedures to guarantee the safety and effectiveness of CAR-T products.
The clinical-grade CDMO services for CAR-T cell therapy are characterized by several key features:
| 1. Production Scale: | At the clinical stage, CDMO services typically involve larger-scale production compared to earlier phases. This is necessary to meet the demands of clinical trials, which often require a significant number of doses for patient administration. |
| 2. Quality Control: | Stringent quality control measures are applied to oversee both the manufacturing process and the final product. This includes testing for cell viability, purity, and functionality to ensure that the CAR-T cells meet the required specifications for clinical use. |
| 3. Technical Expertise: | Clinical CDMO services leverage advanced technologies and methodologies in cell processing, including optimized culture conditions, gene editing techniques, and automated systems to enhance efficiency and consistency in production. |
| 4. Data Collection and Analysis: | During the clinical phase, extensive data collection and analysis are conducted to evaluate the therapeutic effects and safety profiles of the CAR-T cells. This data is essential for regulatory submissions and guiding future treatment protocols. |
| 5. Collaboration with Clinical Trials: | CDMO services at this level often work closely with clinical trial sponsors to align manufacturing capabilities with trial timelines and patient needs, ensuring timely delivery of CAR-T products for administration. |
The clinical-grade CAR-T cell manufacturing services offered by Creative Biolabs are designed to provide comprehensive solutions for cellular therapy products. These services include GMP-compliant manufacturing of CAR-T cells, allowing for customization based on client needs. The manufacturing process takes place in a fully compliant B+A grade cleanroom environment with unidirectional airflow, ensuring adherence to stringent quality management systems. Creative Biolabs also facilitates technology transfer, offering well-structured plans for both transferring and receiving technologies across various phases. Quality control is rigorously maintained through a variety of tests, including assessments of cell viability, CAR positivity, and safety measures such as endotoxin and sterility testing.

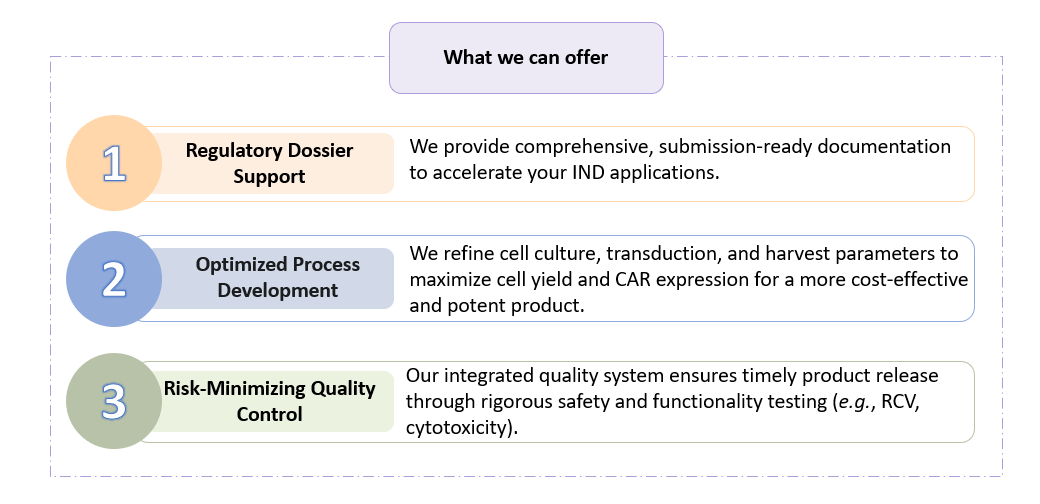
We provide end-to-end solutions that cover the entire therapeutic lifecycle, from raw material procurement to final product release. Our scientific team focuses on process robustness and validation, guaranteeing that every batch meets the most stringent global standards for safety, purity, and potency.
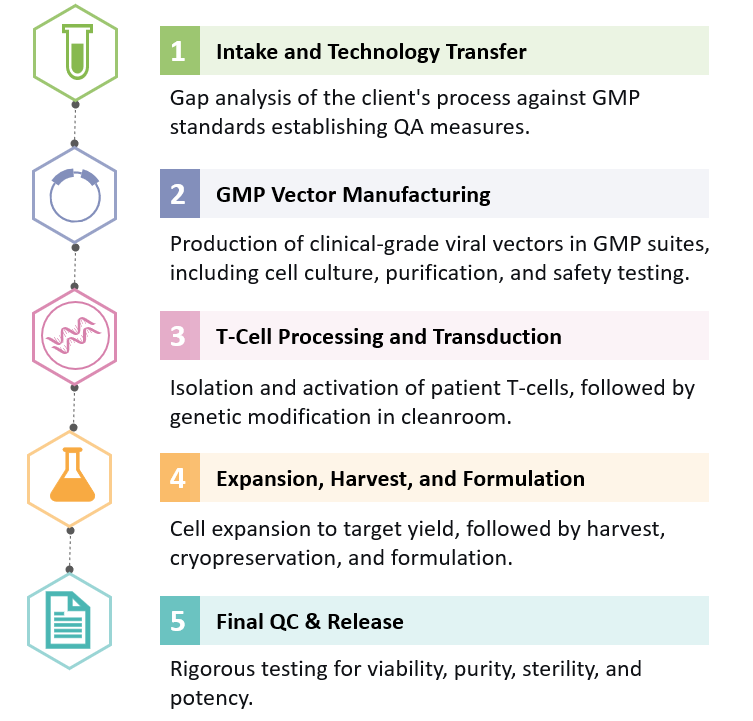
This detailed process highlights the main stages required to translate a research-grade CAR-T concept into a clinical-grade therapeutic, offering full transparency and clarity on the steps involved for potential clients.
How does Creative Biolabs handle the supply chain risk associated with autologous (patient-specific) material?
We use closed-system processing and client-specific protocols to minimize contamination and ensure consistency, supported by strict tracking and identity checks.
Can your service accommodate both viral (Lentivirus, AAV) and non-viral (mRNA / LNP) CAR delivery methods?
Yes. Our platform supports both viral (Lentivirus, Retrovirus, AAV) and non-viral (mRNA / LNP) methods in dedicated GMP suites, offering full flexibility for CAR design.
We are currently using a different CDMO for our vector. Can Creative Biolabs handle only the cell processing part?
Yes. We can accept your client-furnished GMP vector and manage the cell processing, collaborating with your partners to ensure batch success.
Creative Biolabs' service significantly improved the final product yield and purity for our third-generation CAR construct. Their standardized process achieved a fourfold increase in CAR-positive cells, ensuring patient dosing capacity, and provided rapid RCV data. - P**ul W.
The regulatory documentation, especially Batch Documentation and CoA, was exceptional. It greatly facilitated our EMA submission, with their expert CMC data for our Phase II trial proving invaluable. - D**vid M.
Creative Biolabs is your dedicated, full-service CDMO partner, providing robust GMP-compliant Clinical Grade CAR-T Manufacturing Service, backed by two decades of scientific excellence. We deliver high-yield cell products, meticulous quality control, and essential regulatory documentation to accelerate your path to market.
Creative Biolabs is equipped to meet diverse client requirements in the field of CAR-T cell therapy. Contact us, and we would be pleased to provide you with more information and look forward to collaborating with you.
Perhaps you may also be interested in the following Global CDMO Services for CAR Cells:
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION