We support the design and optimization of next-generation CAR-T constructs including tandem CARs for dual-targeting, inhibitory CARs for safety, and logic-gated CARs for improved tumor selectivity.
Immune effector cell-associated neurotoxicity syndrome (ICANS) is one of the most serious complications following CAR-T therapy, often manifesting as seizures, encephalopathy, or aphasia. Its unpredictability and variable onset have challenged developers globally. Are you struggling with unpredictable neurotoxicity, delayed CNS symptoms, or difficulty detecting early warning signs? Creative Biolabs integrates cellular, molecular, and functional screening tools to identify neurotoxicity drivers and reduce failure risks across CAR-T pipelines. Our platform helps you control ICANS risk through multi-layered CNS screening, cytokine profiling, and functional neuroimmune assays.
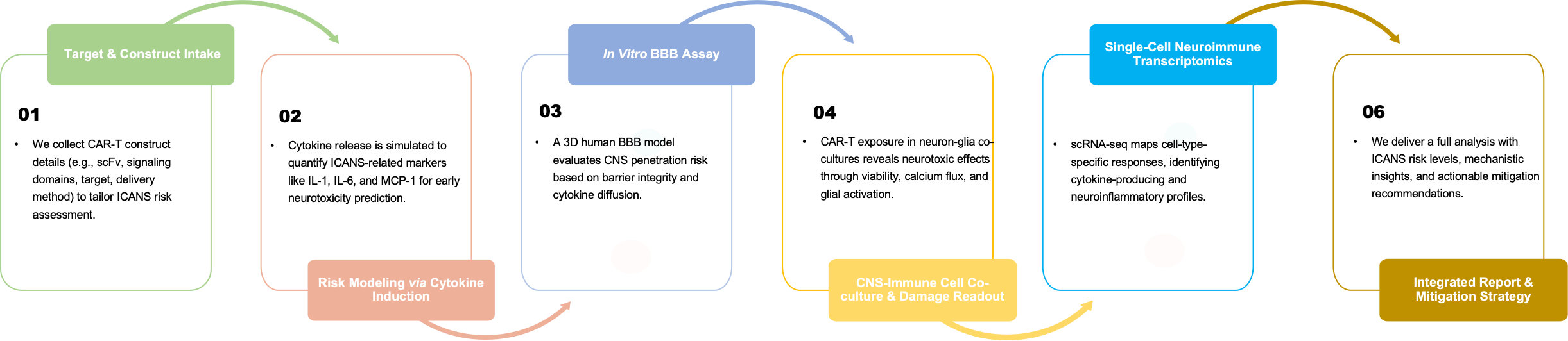
Creative Biolabs delivers a full-service ICANS mitigation workflow including:
We help you prevent costly delays and adverse outcomes by offering mechanistic insight and actionable readouts around ICANS onset. Our services support CAR optimization, safety switch integration, and patient stratification efforts by linking immunotoxic signals to neural outcomes. Whether you're refining a CD19 program or exploring BCMA or GPRC5D targets, our platform identifies CNS liabilities early.
Discover How We Can Help – Request a Consultation
Creative Biolabs employs a comprehensive ICANS control strategy grounded in immunobiology and translational neuroscience:
Our layered approach targets both primary inflammatory triggers and downstream neurological effects to guide mitigation design.
We evaluate cytokine panels (e.g., IL-1, IL-6, MCP-1) and inflammatory signatures to identify early neurotoxicity risks. Analysis informs patient stratification and construct redesign.
Our 3D BBB model assesses CAR or cytokine-induced barrier disruption, supporting CNS penetration risk evaluation and mitigation in early-stage development.
We quantify inflammatory cytokine surges from CAR exposure in neural co-culture models, highlighting immune-mediated risks linked to ICANS pathogenesis.
We map glial and immune transcriptomes at single-cell resolution to uncover pro-inflammatory states and identify early CNS reactivity markers.
Using functional imaging and cytokine profiling, we measure microglia and astrocyte responses under CAR-T exposure, revealing neuroinflammatory toxicity potential.
We integrate CNS-focused cytokine panels, glial activation models, and neuro-immune co-culture systems to deliver a deep understanding of ICANS drivers, helping clients proactively identify and address neurotoxic mechanisms before clinical stages.
Our in vitro blood-brain barrier (BBB) and brain cell co-culture systems are based on human-derived cell types, enabling accurate prediction of CNS responses to CAR-T constructs in physiologically relevant contexts.
We employ single-cell RNA sequencing of immune and neural cells to detect microenvironmental changes, immune infiltration, and neuroinflammation at unprecedented resolution, supporting construct redesign and risk scoring.
Whether you're using lentiviral, mRNA, or non-viral delivery, our ICANS solutions integrate seamlessly with any CAR-T modality and are adaptable to various cell types, including T, NK, and γδ cells.
Experience the Creative Biolabs Advantage – Get a Quote Today
Can your ICANS services be applied to non-CD19 CAR targets?
Yes. Our platform supports CD19, BCMA, GPRC5D, and other targets including solid tumor CARs, tailoring assessments to antigen expression and CNS penetration risk.
How do you simulate CAR-T exposure to the CNS in vitro?
We use human-derived co-culture systems with neurons, astrocytes, and microglia alongside BBB models to replicate CNS inflammatory conditions post-infusion.
What sample inputs are needed for single-cell neurotoxicity profiling?
We can work from in vitro samples, xenograft-derived tissues, or primary glial exposure systems. We'll guide you on format and preparation.
Is the service modular or all-in-one?
Our ICANS solutions are fully modular—select from cytokine panels, BBB testing, scRNA-seq, or glial activation as needed for your development stage.
We support the design and optimization of next-generation CAR-T constructs including tandem CARs for dual-targeting, inhibitory CARs for safety, and logic-gated CARs for improved tumor selectivity.
Using IHC, RNAseq, and proteomics, we help clients identify tumor-specific surface antigens while minimizing off-tumor expression, enabling safer and more effective CAR target selection strategies.
Creative Biolabs provides trusted ICANS mitigation strategies across the CAR-T lifecycle—from early discovery to clinical submission. Our tools accelerate development, reduce neurotoxicity risk, and enhance therapeutic confidence.
Explore Our End-to-End CAR-T Development Programs – Ask Us How
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION