CAR-T therapy has emerged as a groundbreaking approach in the treatment of various cancers, leveraging the body's immune system to target and eliminate malignant cells. As the demand for CAR-T therapies continues to grow, the need for reliable and efficient Contract Development and Manufacturing Organization (CDMO) services becomes increasingly critical. Creative Biolabs stands at the forefront of this field, offering a comprehensive suite of global CDMO services tailored for CAR cells, ensuring high-quality, customized solutions that meet the diverse needs of clients across different stages of development.
| Category | IIT Grade CAR-T Manufacturing | IND Grade CAR-T Manufacturing | Clinical Grade CAR-T Manufacturing |
|---|---|---|---|
| Purpose | Designed for investigator-initiated or academic research studies. | Intended for preclinical evaluation and early human studies requiring regulatory preparation. | Developed for formal clinical studies and large-scale therapeutic applications. |
| Quality System | Conducted under controlled laboratory or GMP-like conditions with institutional oversight. | Manufactured under enhanced quality management with full traceability and standardized documentation. | Produced in certified facilities under strict quality assurance systems for human application. |
| Testing & Control | Basic in-process testing to verify sterility, viability, and CAR expression. | Expanded quality testing, including identity, purity, safety, and functional activity. | Comprehensive testing with validated analytical methods for product consistency, potency, and release criteria. |
| Documentation | Limited documentation for internal use and research records. | Complete batch records, certificates, and production data supporting regulatory submission. | Full documentation package covering manufacturing, validation, and product release. |
| Production Scale | Small-scale preparation suitable for laboratory or proof-of-concept studies. | Intermediate scale supporting pilot or early-phase trials. | Large-scale, quality-controlled production for advanced clinical evaluation or commercial readiness. |
| Timeline | Rapid production cycle enabling quick experimental turnaround. | Moderate production timeline with extended quality testing. | Longer timeline due to extensive validation and release procedures. |
Creative Biolabs provides three primary tiers of CAR-T manufacturing services. Each of these services is designed to adhere to stringent regulatory requirements while ensuring the safety and efficacy of CAR-T products.
IIT Grade CART Manufacturing focuses on providing integrated CDMO services specifically tailored for early-phase clinical trials. This includes dossier preparation for ethical and regulatory approvals, process development, and validation, all conducted in a GMP-like environment. Creative Biolabs employs advanced automated culturing equipment to enhance cell proliferation and maintain storage and shipping stability, ensuring that CAR-T cells are produced efficiently and safely. The emphasis on cryopreserved cell preparation and automated systems aligns with global industry standards, facilitating high-quality production.
IND Grade CART Manufacturing encompasses a broader range of services, including the development of tailored processes and test methods that comply with GMP standards. This stage is crucial for ensuring that CAR-T cells are produced at scalable levels while maintaining high quality. Creative Biolabs conducts rigorous quality control measures, including stability studies and safety assessments, to validate the manufacturing methods. The use of closed and automated cell culture systems further enhances efficiency and consistency, addressing challenges related to low positive rates and proliferation rates.
Clinical Grade CART Manufacturing is characterized by larger-scale production necessary for clinical trials, which often require significant doses for patient administration. Creative Biolabs ensures that all manufacturing processes are conducted in a fully compliant cleanroom environment, adhering to stringent quality management systems. The services include extensive data collection and analysis to evaluate the therapeutic effects and safety profiles of CAR-T cells, which are essential for regulatory submissions and future treatment protocols. Collaboration with clinical trial sponsors is also a key feature, ensuring that manufacturing capabilities align with trial timelines and patient needs.
Creative Biolabs offers a robust portfolio of global CDMO services for CAR cells, encompassing IIT, IND, and clinical grade manufacturing. With a commitment to quality, safety, and efficiency, Creative Biolabs is well-equipped to support the advancement of CAR-T therapies.

By leveraging advanced technologies and methodologies, we aim to facilitate the successful implementation of CAR-T cell therapies, ultimately contributing to improved treatment outcomes for battling cancer. For more information or to explore partnership opportunities, Creative Biolabs invites interested parties to reach out and collaborate in this vital field of cellular therapy.
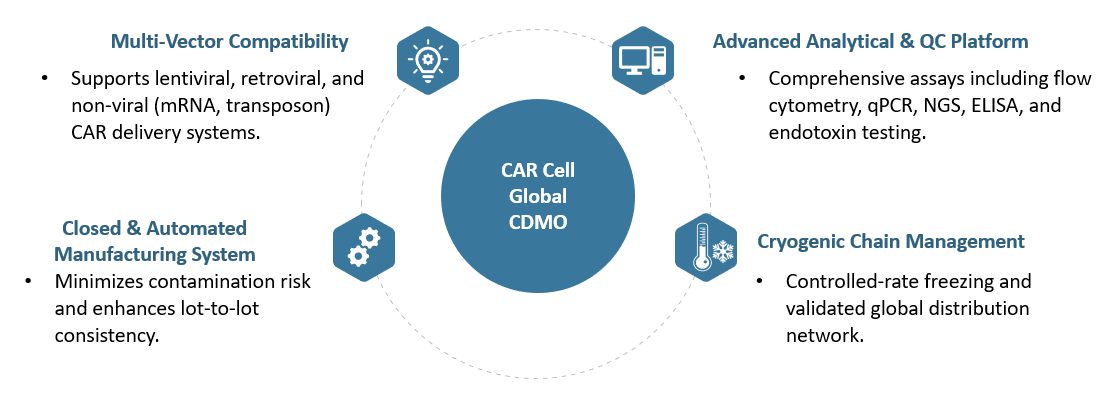
Our technology platforms are designed to meet the highest standards of reproducibility, scalability, and regulatory compliance.
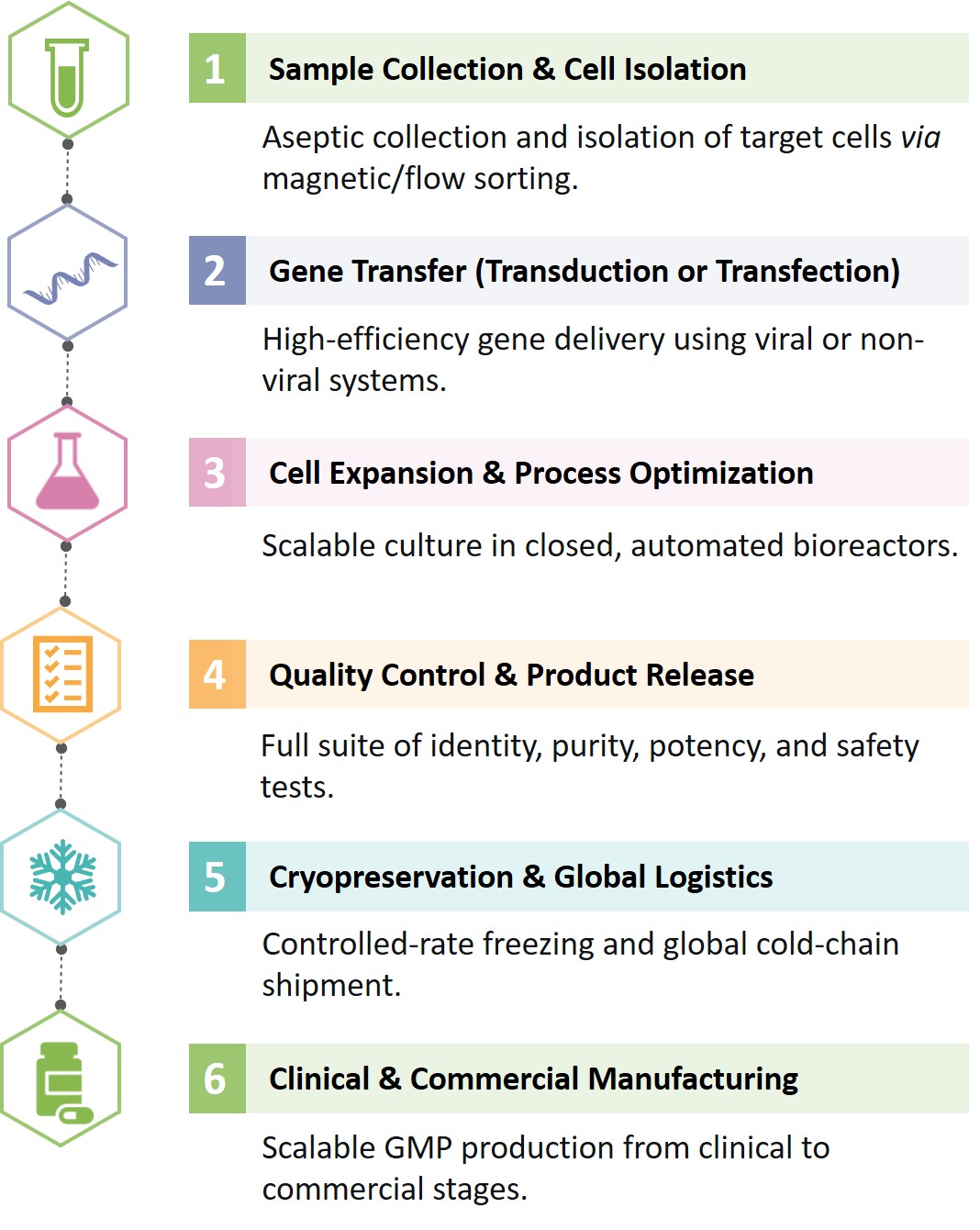
We deliver a streamlined end-to-end process — from sample acquisition to GMP manufacturing — ensuring consistency and compliance at every step.
Connect with our team for additional information and to go over your project.
What are the differences between IIT, IND, and Clinical Grade production?
IIT grade supports early investigator-initiated research, IND grade meets preclinical regulatory standards, and Clinical grade ensures full GMP compliance for large-scale production.
What quality standards do you follow?
Our facilities operate under GMP guidelines, with full QA/QC documentation and batch traceability.
Can you handle both viral and non-viral CAR systems?
Yes. We provide complete process development and manufacturing solutions for viral (LV, RV) and non-viral (mRNA, transposon) platforms.
Do you provide technology transfer and long-term partnership options?
Absolutely. We offer flexible technology transfer, co-development, and long-term supply models tailored to client needs.
"Creative Biolabs delivered outstanding technical and regulatory support throughout our CAR-T development. Their GMP manufacturing quality exceeded expectations."— Director of Cell Therapy R**D.
"The closed-system manufacturing platform significantly improved our cell yield and consistency. Communication and project management were seamless."— Senior Project Manager P**E.
"Fast turnaround, reliable QC data, and comprehensive documentation — a trusted CDMO partner for our clinical pipeline."— Principal Investigator, A***H.
At Creative Biolabs, we are more than a CDMO — we are your strategic partner in advancing CAR cell therapy from research to commercialization. Our global infrastructure, expert teams, and proven track record make us a trusted choice for innovators worldwide. Contact us today to discuss your project needs and discover how our CAR cell CDMO platform can accelerate your path to clinical and commercial success.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION