CAR T-cell therapy, a revolutionary approach that genetically engineers a patient's T-cells to target and destroy cancer cells, has demonstrated remarkable efficacy, particularly in B-cell malignancies. Despite its success, the therapy can induce severe systemic inflammatory responses, leading to a range of toxicities. Among these, Cytokine Release Syndrome (CRS) and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS) are well-recognized. Less distinctly characterized, yet critically important, is the associated coagulopathy, which can manifest as a spectrum from subclinical laboratory abnormalities to life-threatening hemorrhagic or thrombotic events.
The development of coagulopathy in CAR T-cell recipients is often intertwined with the severity of CRS. The massive release of cytokines during CRS can activate endothelial cells and leukocytes, leading to a procoagulant state that may progress to a disseminated intravascular coagulation (DIC)-like picture. Therefore, a nuanced understanding and proactive management of coagulopathy are indispensable components of comprehensive CAR T-cell therapy protocols. At Creative Biolabs, we recognize the critical need for refined strategies to address these hematological complications.
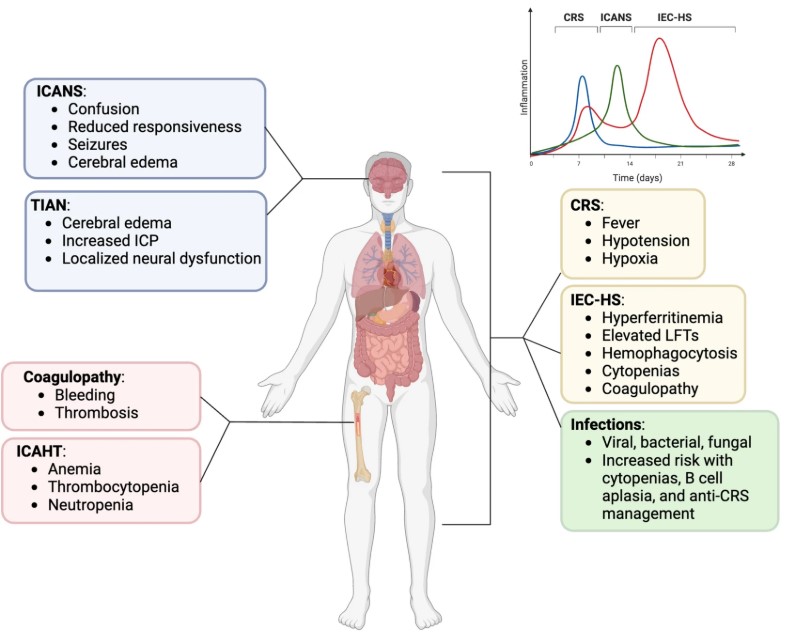 Fig.1 Schematic diagram of CAR T-cell toxicities.1
Fig.1 Schematic diagram of CAR T-cell toxicities.1
Given the potential for rapid evolution of coagulopathy, particularly in patients experiencing moderate to severe CRS, vigilant laboratory monitoring is crucial. Creative Biolabs supports these critical monitoring needs by providing advanced services. This typically includes:
D-dimer: D-dimer is a specific fibrin degradation product (FDP) that is formed when cross-linked fibrin clots are broken down by plasmin. Elevated D-dimer levels in the blood are an indicator of increased fibrinolysis, which typically occurs secondary to active coagulation and thrombin generation (thrombosis). In the context of CAR T-cell therapy, significantly elevated D-dimer levels can signal a prothrombotic state, the presence of ongoing clot formation and breakdown, and may be suggestive of developing or established Disseminated Intravascular Coagulation (DIC).
Creative Biolabs' D-Dimer Level Testing service offers a reliable and sensitive method for quantifying D-dimer concentrations. This testing aids in the assessment of thrombotic risk and can be a valuable component in the diagnostic workup for DIC. Monitoring D-dimer trends can help clinicians evaluate the extent of coagulation activation and the response to interventions aimed at managing the underlying CRS and its coagulopathic complications.
Fibrinogen Levels: Fibrinogen (Factor I) is a soluble glycoprotein synthesized by the liver that plays an essential role in the coagulation cascade; it is converted by thrombin into insoluble fibrin strands, which form the structural meshwork of a blood clot. Due to its central role and early alteration in CAR T-cell therapy (often consumed or its production impaired during CRS-induced inflammation), fibrinogen is a critical marker of coagulopathy. Baseline measurements and frequent monitoring (e.g., daily or more often in high-grade CRS) are recommended to detect hypofibrinogenemia promptly.
Creative Biolabs' Fibrinogen Level Assay service provides precise, quantitative measurement of circulating fibrinogen levels. This service utilizes advanced methodologies to ensure accuracy and reproducibility, enabling clinicians to make informed decisions regarding the need for fibrinogen replacement therapy (e.g., cryoprecipitate or fibrinogen concentrate) and to assess the response to such interventions. Timely and accurate fibrinogen assessment is vital for mitigating bleeding risks associated with severe hypofibrinogenemia in CAR T-cell recipients.
At Creative Biolabs, with over two decades of expertise in biological sciences and therapeutic innovation, we are acutely aware of the challenges posed by CAR T-cell therapy-associated toxicities. Our commitment extends beyond understanding these complexities to actively developing and providing solutions that enhance patient safety and therapeutic efficacy.
Q1: What is CAR T-cell therapy-associated coagulopathy?
A1: CAR T-cell therapy-associated coagulopathy refers to a range of blood clotting disorders that can occur as a side effect of the treatment. It is often linked to Cytokine Release Syndrome (CRS) and is commonly characterized by low levels of fibrinogen (hypofibrinogenemia), a key protein for blood clotting. This can increase the risk of bleeding, though thrombotic events can also occur.
Q2: Why is monitoring fibrinogen levels particularly crucial in patients receiving CAR T-cell therapy?
A: Fibrinogen levels can drop significantly and disproportionately during CAR T-cell therapy, especially with higher grades of CRS. Low fibrinogen is a direct indicator of coagulopathy severity and correlates with an increased risk of bleeding. Frequent monitoring allows for timely intervention, such as fibrinogen replacement, to prevent or manage bleeding complications.
Q3: What is the role of managing Cytokine Release Syndrome (CRS) in preventing or treating coagulopathy associated with CAR T-cell therapy?
A3: Cytokine Release Syndrome (CRS) is a major driver of coagulopathy in CAR T-cell therapy. The intense systemic inflammation during CRS activates the coagulation system and can lead to consumption of clotting factors like fibrinogen. Therefore, effectively managing CRS with therapies such as IL-6 inhibitor or corticosteroids can significantly mitigate the severity of associated coagulopathy by reducing the underlying inflammatory stimulus.
At Creative Biolabs, we are committed to advancing the field of cellular immunotherapy through innovative solutions and collaborative partnerships. For further information on our CAR-T toxicity management solutions or to discuss how Creative Biolabs can support your CAR T-cell therapy initiatives, please reach out to our expert team.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION