High-quality isolation and differentiation of dendritic cells from various sources.
Lengthy drug development cycles and robust immune responses limit the development of biochemistry and biopharmaceuticals. To address these challenges, Creative Biolabs provides a series of advanced ex vivo dendritic cell (DC) vaccine design & optimization services to accelerate immunotherapeutic discovery, obtain high-quality antigen-specific immune cells, and streamline preclinical development through advanced cell engineering and precision antigen loading technologies.
Despite immunotherapy's advancements, developing potent, durable T-cell vaccines for complex diseases like cancer and chronic infections remains challenging. Traditional vaccines often fail to activate the precise cellular immunity needed. Dendritic cells (DCs), as key antigen-presenting cells (APCs), are crucial for initiating immune responses. Utilizing DCs in vaccine design offers a powerful, targeted approach to prime naïve T cells and drive robust anti-tumor or anti-viral immunity, overcoming current limitations. This approach is vital for gene therapy and personalized medicine, where engineered cellular products provide unprecedented therapeutic precision.
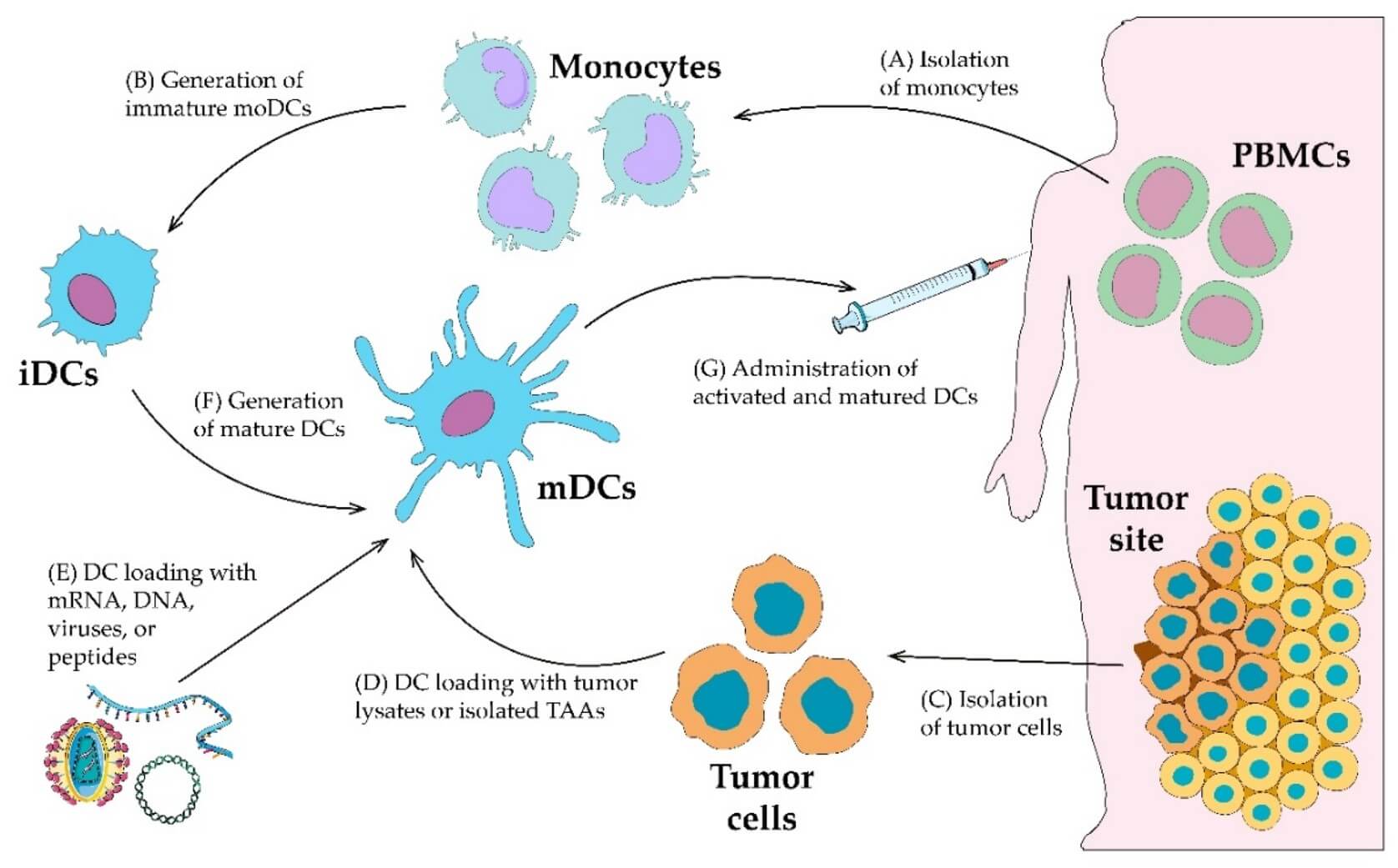 Fig.1 Ex vivo DC vaccine generation.1
Fig.1 Ex vivo DC vaccine generation.1
Creative Biolabs' advanced ex vivo DC vaccine design & optimization services provide comprehensive solutions for developing highly potent and precisely engineered DC vaccines in vitro. Our approach is founded on the principle of precisely manipulating isolated patient or donor immune cells, specifically monocytes, to differentiate them into immature and then mature dendritic cells. These DCs are then loaded with target antigens and further engineered using cutting-edge techniques like gene editing to enhance their antigen-presenting and immune-stimulating capabilities.
We deliver fully characterized, functional DC vaccine products designed to meet specific project requirements, from fundamental research to preclinical validation. Our typical project timeline ranges from 6 to 12 weeks, depending on complexity. The quality of our results is rigorously assured through comprehensive analyses of DC phenotype, viability, purity, sterility, and critical functional assays, ensuring you obtain high-quality, antigen-specific immune cell products. Our services are tailored to accelerate your immunotherapeutic development.
We begin by isolating target immune cell populations, typically monocytes from provided leukapheresis products. These monocytes are then meticulously differentiated into immature dendritic cells (iDCs) under controlled conditions, commonly using specific cytokines such as GM-CSF and IL-4, to ensure high purity and viability.
The differentiated iDCs are then pulsed with your specific target antigens. For enhanced efficacy and precision, we offer advanced engineering options:
Following antigen loading and engineering, the iDCs are induced to mature into fully activated, potent mature dendritic cells (mDCs) using specific maturation cocktails (e.g., pro-inflammatory cytokines, TLR ligands). This step is critical for upregulating co-stimulatory molecules and MHC expression, ensuring robust T-cell priming capacity.
Throughout the process, and especially at the final stage, stringent quality control measures are implemented. This includes comprehensive analysis of DC phenotype (surface marker expression via flow cytometry), viability, purity, sterility, and critical functional assays (e.g., mixed leukocyte reaction, cytokine secretion assays, antigen-specific T-cell activation assays) to confirm potency.
The fully characterized and optimized DC vaccine product is then prepared according to your specifications, suitable for downstream applications such as preclinical studies or further development.
Creative Biolabs offers comprehensive service packages from DC production to DC vaccine development to cater to various research and development needs:
High-quality isolation and differentiation of dendritic cells from various sources.
DCs loaded with specific antigens to elicit targeted immune responses.
DCs modified using advanced gene editing for enhanced functionality.
Efficient delivery of mRNA antigens into DCs via optimized LNP technology.
Development of DCs pulsed with a wide range of tumor antigens for comprehensive anti-cancer immunity.
Generation of hybrid cells combining DC antigen-presenting capabilities with tumor antigen expression.
Q1: What types of antigens can be used with your DC vaccine services?
A1: Our services are highly versatile and can accommodate a wide range of antigens, including synthetic peptides, recombinant proteins, tumor cell lysates, plasmid DNA, and mRNA sequences encoding specific target antigens. We can also assist in optimizing the antigen presentation strategy for your specific project.
Q2: How do you ensure the quality and safety of the ex vivo DC vaccines?
A2: We employ stringent quality control (QC) measures at every stage of the development process. This includes comprehensive analysis of DC purity, viability, phenotype, and sterility. We also conduct rigorous functional assays, such as T-cell activation assays, to ensure the potency and efficacy of the final DC vaccine product.
Q3: Can your services be tailored for personalized cancer vaccines?
A3: Absolutely. Our services are ideally suited for personalized cancer vaccine development. We can integrate patient-specific tumor antigen identification, such as neoantigen prediction, into our workflow to create highly individualized DC vaccines designed to target unique tumor specificities. We encourage you to discuss your specific personalized medicine project with our experts.
Creative Biolabs is dedicated to advancing the field of immunotherapeutics through our unparalleled scientific expertise and cutting-edge technologies. Our advanced ex vivo dendritic cell (DC) vaccine design & optimization services are designed to accelerate your research, overcome developmental hurdles, and ultimately bring effective therapies to patients faster. Partner with us to leverage our years of experience and innovative solutions for your next breakthrough.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION