The development and manufacture of high-quality plasmid DNA for Chimeric Antigen Receptor T-cell (CART) therapy are critical due to the therapy's targeted and systemic nature. In the continually evolving field of genetic engineering, ensuring the quality, purity, and scalability of plasmid DNA is paramount. With decades of experience, Creative Biolabs facilitates advanced, research-grade plasmid DNA manufacturing techniques tailored for CART applications.
The integrity of the plasmid DNA is the foundation of all gene and cell therapy modalities, particularly CAR-T. A high-quality plasmid is essential as it dictates the efficiency of gene delivery and the eventual function of the engineered T-cell. Creative Biolabs' RG service focuses on manufacturing plasmid DNA with exceptional purity and precise quality control, making it ideal for target validation, CAR construct optimization, and early preclinical studies.
Our RG Plasmid DNA Manufacturing Service is a direct solution to the inconsistency and contamination challenges inherent in early-stage CAR-T development. We provide high-quality starting material that ensures reliable, reproducible experimental results, paving a smoother path to regulatory filings.
Clients leverage our service to reliably express their CAR or other gene therapy constructs. We solve the critical early-stage problem of variability in T-cell engineering efficiency caused by low-purity, contaminated, or improperly structured plasmid DNA. Our high-purity, supercoiled DNA minimizes off-target effects and maximizes viral packaging and cell transduction yields. This reliability directly translates to faster in vitro and in vivo validation studies.
Connect with our team members for further information and project discussion.
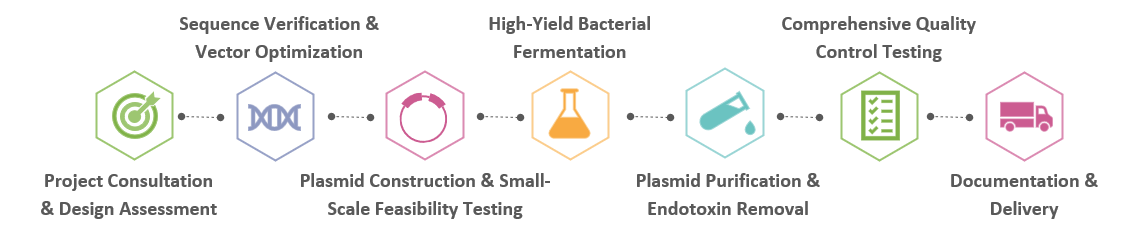
Creative Biolabs offers a comprehensive suite of services for RG plasmid DNA manufacturing, designed to support a wide range of experimental needs:
Plasmid Engineering and OptimizationTailor-made DNA plasmid sequences designed for optimal expression and functionality in CART applications.
From microgram to gram scales, our facilities are equipped to handle varying production scales to match project requirements.
Using advanced chromatographic techniques to achieve high purity levels with minimal endotoxin content.
Rigorous quality control measures are applied at every stage of production to ensure the highest standards are maintained.
Required Starting Materials:
Ensuring the quality and consistency of RG plasmid DNA is paramount for the success of CART therapies. At Creative Biolabs, our quality control protocols include:
Every batch undergoes thorough visual analysis to detect any inconsistencies or contaminants.
Nanodrop technology is employed to accurately determine plasmid concentration.
Endotoxin levels are quantified using kinetic chromogenic LAL assays to meet stringent regulatory standards (<0.05 EU/μg for RG grade).
Agarose gel electrophoresis is used to ensure high proportions of supercoiled plasmid forms, essential for effective CART transfection.
Sanger sequencing or Next-Generation Sequencing (NGS) are available options for confirming plasmid identity and integrity.
Creative Biolabs' services stand out due to several key advantages:
How do you ensure the purity of RG plasmid DNA?
Creative Biolabs utilizes a combination of selective precipitation, advanced chromatographic purification, and rigorous quality control measures to ensure the highest levels of plasmid purity. Every batch undergoes thorough testing to verify purity.
Can you handle large-scale plasmid production?
Yes, our facilities are equipped to scale production from micrograms to multi-gram quantities, accommodating the needs of larger preclinical and research projects.
Creative Biolabs remains dedicated to advancing the field of CART therapies through the provision of high-quality Research Grade (RG) plasmid DNA. Our extensive expertise, state-of-the-art facilities, and commitment to excellence enable us to support cutting-edge research and development projects, driving innovation and improving therapeutic outcomes in the fight against cancer.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION