miRNA in Metabolic Disorders-Fine-Tuning Metabolic Pathways
Introduction of miRNA in Metabolic Disorders
Spatiotemporal expression of miRNAs (short single stranded non-coding RNAs from 18 to 25 nucleotides long) are also seen in eukaryotes, which play an important role in post-transcriptional gene regulation. MiRNAs function as negative regulators of gene expression by binding to their complementary sequence in the 3' or 5' untranslated region (3'UTR or 5'UTR) of the target mRNA and preventing the translation machinery and cleavage of the mRNA. 4571 human miRNAs (1917 precursors and 2654 mature) have been listed in public databases and the number of non-coding RNAs is estimated to regulate ~30% of protein-coding genes that participate in many biological processes. Human miRNA tissue atlas is a database of miRNAs cataloguing the tissue-specific distribution and expression of miRNAs. It provides researchers with an opportunity to explore the physiological and pathological effects of miRNAs. Several miRNAs have recently been identified to be involved in the biology of adipose tissue (development and metabolism), insulin secretion and action, and therefore, may contribute to the development of obesity and associated metabolic complications. Some examples include miR-14, miR-278 and let-7, which are involved in lipid and glucose metabolism.
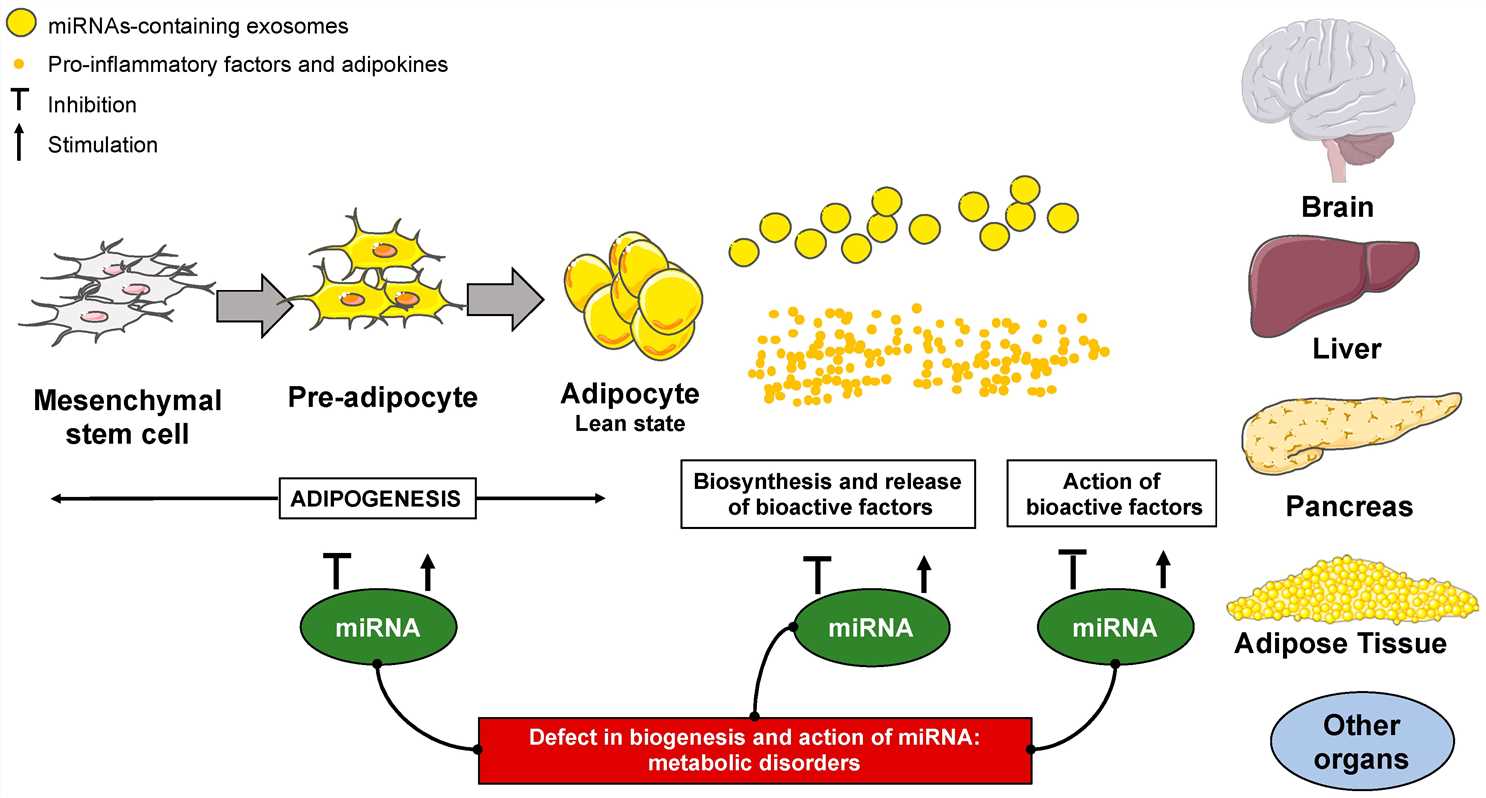 Fig. 1 The functions of micro RNAs (miRNAs) in the adipose tissue development and functions1,6.
Fig. 1 The functions of micro RNAs (miRNAs) in the adipose tissue development and functions1,6.
What are the Metabolic Disorders
A metabolic disorder or metabolic disease is any condition that causes an abnormal chemical reaction in the metabolism process of the body. At the cellular level, a metabolic disorder may describe the process of turning food into energy. Metabolic disorder symptoms arise when the body is in a state of metabolic stress. Most metabolic disorders affect more than one metabolic pathway, and thousands of enzymes act in an interdependent manner. Metabolic diseases can inhibit a cell's ability to perform many different biochemical reactions necessary for a number of processes, including protein transport (amino acids), carbohydrate transport (sugars and starches), or lipid transport (fatty acids).
Delivery Systems for miRNA applications in Metabolic Disorders
- Virus-Based miRNA Delivery Systems
The lentivirus genus in the Retroviridae family has produced a range of immunodeficiency viruses including bovine (BIV), feline (FIV), equine (EIAV), simian (SIV), and human (HIV-2) which scientists use to develop lentiviral vectors (LVs). RVs require nuclear membrane destruction during mitosis to integrate into the host chromosome while LVs pass through intact nuclear membranes via nuclear pores to target both active and inactive cells. In addition, there is a significant risk of insertional oncogenesis when using RVs. As LVs integrate into the actively transcribing regions, the risk of insertional oncogenesis is lessened. Many studies have used LVs to deliver therapeutic miRNA mimics or antagonists. LV-delivered miR-15a and miR-16 in a mouse model of chronic lymphocytic leukemia (CLL), resulting in depletion of malignant B cells and reduced disease. The LV-delivered miR-494 sponge was tested in treatment of leukemia. Results indicated that miR-494-conjugated anti-miRNAs were inhibiting them from binding to cellular proteins, thereby arresting tumor progression and metastasis. Adenoviruses (Ad) and adeno-associated viruses (AAV) are non-enveloped viruses with double-stranded and single-stranded DNA genomes, respectively. An AAV delivery system with high transduction efficiency was used to overexpress miR-298 and alleviated neuromuscular diseases in mice.
- Non-Viral-Based miRNA Delivery Systems
The most commonly used non-viral delivery systems based on lipids are lipid-based nanocarriers. First, cationic lipids with hydrophilic head and hydrophobic tails form a complex with the anionic nucleic acid and form a lipoplex. These cationic lipoplexes are bound to the cell membrane with high affinity, which are non-immunogenic and easily prepared. Many commercial cationic lipoplexes such as Lipofectamines are widely used for miRNA delivery. Cationic liposomes have been used to deliver miRNA in vivo, but their delivery efficiency is relatively low. Some methods were modified to address this issue. Conjugating a polyethylene glycol (PEG) functional group to the cationic lipids can evade phagocytosis and improve the efficiency overall. A report showed that PEG-fused liposomes successfully delivered miR-126 and promoted blood flow and angiogenesis in a hindlimb ischemia model. Other studies have shown the successful in vivo delivery of lipoplexes such as systemic delivery of miR-29b fused with DOTMA, cholesterol, and PEG in NSCLC cells. Covalent conjugation of PEG or poly L-Lysine (PLL) to PEI can increase its biocompatibility and reduce cytotoxicity. A PEG/PEI nanocomplex polymeric vector has been found to be stable and effective in transfecting miR-150 in human leukemia cells.
MiRNA roles in Metabolic Diseases
- MiRNAs in Obesity
An increased expression of miR-221 in the adipose tissue and liver of leptin deficient ob/ob. Different mouse models of obesity can be used as appropriate models to evaluate whether one miRNA is involved in obesity. As mentioned above, miR-21 was up-regulated in obese humans. In a different approach, the group of S. Dimmeler demonstrated that locked nucleic acid (LNA)-miR-21 treatment led to a significant weight loss and reduction in adipocyte size, as well as repression of targets such as TGFβ-receptor 2 (TGFBR2) and phosphatase and tensin homolog (PTEN). Similarly, let-7 knockout mice did not develop insulin resistance. In the same way, it has been shown that weight loss regulated the circulating levels of miRNAs. For example, a number of miRNAs were plasma levels similar to those of lean controls after acute weight loss in women who are obese.
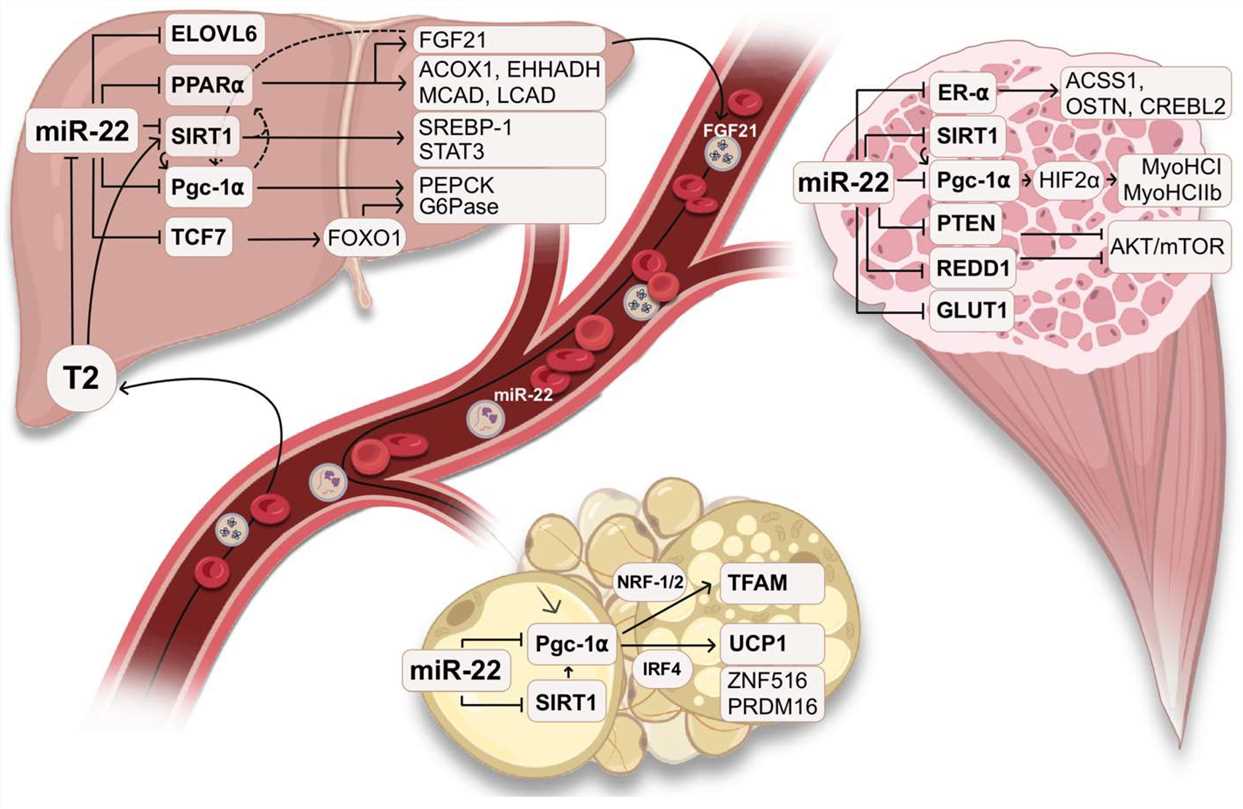 Fig. 2 The physiological role of miR-22 in metabolism2,6.
Fig. 2 The physiological role of miR-22 in metabolism2,6.
- The Role of MiRNAs in Adipogenesis
Studies involving Drosophila flies revealed that miR-14 and miR-278 control lipid metabolism which provided initial evidence for miRNA influence on adipogenesis. Many miRNAs have been implicated in adipogenesis. In particular, the effects of miR-143 have been investigated in detail. The human preadipocytes that transfect antisense oligonucleotides of miR-143 have shown altered adipocyte differentiation. The research indicated that miR-143 levels in obese mice's adipose tissues matched the expression patterns of the adipocyte differentiation marker PPARγ. The miRNA cluster miR-17-92 demonstrated increased expression during adipocyte clonal expansion and accelerates adipocyte differentiation by targeting the cell cycle regulator Rb2/p130. Several miRNAs target C/EBP during adipogenesis. For example, miR-375 stimulates 3T3-L1 adipocyte cell line differentiation by up-regulating the expression of C/EBP and PPARγ2. It has been shown that the correlation between miR-519d and the protein levels of PPARα involved in fatty acid homeostasis. In the case of miR-519d, the translational activity of the PPARα protein was suppressed, and the lipid accumulation during preadipocyte differentiation was increased.
- The Inflammation of the Adipose Tissue and MiRNAs
Many studies have reported that the expression of miRNAs is dysregulated during adipose tissue inflammation. An overrepresented miRNA expression pattern has been identified in the TNF-α-treated 3T3-L1 adipocyte cell line and in adipocytes from the leptin-deficient ob/ob mice. Decreased miR-221 expression by TNF-α or leptin was also observed in human preadipocyte. Increased miR-335 expression by TNF-α was observed in murine adipocyte. Expression of miR-130, miR-146a, miR-146b, miR-150, miR-221, miR-222 and expression of miR-103 and miR-143 were changed by TNF-α in murine adipocyte. Expression of miR-221 in human adipose tissue-derived mesenchymal stem cells isolated from an obese woman with high expression of TNF-α. miR-145 induced the expression of TNF-α via NF-κB pathway in adipocytes. Interestingly, researchers identified a proinflammatory link between NF-κB and miR-155 which may contribute to the inflammation status amplification in adipocytes.
- The Role of miRNAs in Insulin Synthesis, Secretion and Sensitivity
Type 2 diabetes (T2D) is a condition that occurs when there is both decreased insulin secretion and reduced insulin sensitivity. Several miRNAs have been identified as β-cell specific and were first discovered in a pancreatic endocrine cell line. MiR-375 has been shown to decrease β-cell number and viability after overexpression in β-cells. MiR-375 regulates insulin secretion by modulating the expression of Aifm1, Gphn, ywhaz and MTPN which are critical for exocytosis. Insulin exocytosis inhibition happens through miR-9 targeting both the transcription factor one cut homeobox 2 (Onecut2) and granuphilin (Sytl4), which negatively regulates secretion. miR-124a and let-7b, which are also expressed in pancreatic islet cells, have been shown to regulate insulin release by targeting MTPN expression.
miRNA therapeutics in Metabolic Disorders
The advantage of targeting miRNAs is that it can block several metabolic genes at once instead of blocking single genes. One highly abundant liver-specific miRNA in metabolism is miR-122, which is involved in cholesterol and lipid metabolism. Antisense inhibitors of miR-122 reduced viral titers. The antisense approach targeting miR-103/107 boosted insulin sensitivity and glucose uptake in obese mouse models which could provide therapeutic benefits for insulin resistance caused by obesity. miR-34a is a negative regulator of the key lipid and insulin signaling gene SIRT1 and is involved in Non-alcoholic fatty liver disease. The most popular application of miRNA-based therapeutics is antisense oligonucleotides (ASOs). Use of ASOs with LNA-modified oligonucleotides decreases the level of endogenous miRNAs and increases the expression of target genes. For example, antisense targeting of miR-33 increased high density lipoprotein cholesterol levels and decreased atherosclerotic plaque size. Synthetic miRNA mimics restore the expression of downregulated miRNAs.
Future Directions
MiRNAs are attractive targets for intervention in metabolic disorders in various metabolic organs and may be important for breaking or mediating the metabolic crosstalk necessary for the specific supply of metabolic health. Based on the description above, it is clear how much work is required to fully understand the function of a single miRNA in controlling animal physiology. But with the help of new technologies combining RNA-sequencing, proteomics and systems biology technologies, we will be able to understand better how the control of metabolic processes is affected by the modification of gene networks by miRNAs. As the significance of miRNAs in each disease becomes more and more understood and established, we will certainly see the development of cutting-edge tools for detection and treatment of major human diseases. In short, it seems that this new knowledge will soon be an important part of the practice of personalized medicine.
References
- Landrier, Jean-François, Adel Derghal, and Lourdes Mounien. "MicroRNAs in obesity and related metabolic disorders." Cells 8.8 (2019): 859. https://doi.org/10.3390/cells8080859.
- Tomasini, Simone, et al. "The Role of microRNA-22 in Metabolism." International Journal of Molecular Sciences 26.2 (2025): 782. https://doi.org/10.3390/ijms26020782.
- Fodor, Adriana, et al. "MicroRNAs: the link between the metabolic syndrome and oncogenesis." International Journal of Molecular Sciences 22.12 (2021): 6337. https://doi.org/10.3390/ijms22126337.
- Sethupathy, Praveen. "The promise and challenge of therapeutic microRNA silencing in diabetes and metabolic diseases." Current diabetes reports 16 (2016): 1-6. https://doi.org/10.1007/s11892-016-0745-3.
- MacDonald-Ramos, Karla, et al. "Effect of dietary fatty acids on MicroRNA expression related to metabolic disorders and inflammation in human and animal trials." Nutrients 13.6 (2021): 1830. https://doi.org/10.3390/nu13061830.
- Distributed under Open Access license CC BY 4.0, without modification.
