miRNA in Neurodegenerative Diseases-The Microscopic Regulators
Introduction of miRNA in Neurodegenerative Diseases
MicroRNAs (miRNAs) are a group of short non-coding RNAs involved in gene expression during development, differentiation and in response to environmental stress. MiRNAs have been found to regulate the expression of the genes associated with the pathogenesis of neurodegenerative diseases (NDs). The target mRNAs are downregulated by binding of miRNAs to the complementary sequence in the 3' untranslated region (3' UTR) and cause degradation or translational repression of the target mRNAs leading to reduced expression of the protein. The same miRNA can target multiple mRNAs and the same mRNA can be targeted by multiple miRNAs; several miRNAs together can target a single mRNA and this will allow the fine-tuning of the expression of a gene network. In NDs such as Alzheimer's disease (AD), Parkinson's disease (PD) and Huntington's disease (HD), miRNAs regulate the expression of the proteins associated with the pathogenesis of these diseases.
The urgency of treating of Neurodegenerative Diseases
NDs are characterized by a selective loss of neurons, which normally results in death. They include progressive neuropsychiatric diseases, e.g., AD, Multiple sclerosis (MS), PD, Amyotrophic lateral sclerosis (ALS), HD, and other NDs. NDs are typically associated with a slow but continuous loss of neurons and synapses and usually occur in late life. Characteristic clinical symptoms are usually recognizable and caused by distinct brain areas with massive neuronal loss. In ADs, for instance, neuronal loss can be seen early in the hippocampus, a region crucial for declarative episodic memory. In PD, the cardinal clinical trial for tremor, bradykinesia and postural instability only becomes manifest after a massive loss of 70–80 % of dopaminergic neurons in the substantia nigra. In MS activated immune reactions (microglia) of the brain attack the myelin sheaths of the neurons that will ultimately result in demyelination and the conduction difficulties of neuronal signals. In 2019, major neurological disorders resulted in 10 million deaths and affected 349.2 million individuals globally. These disorders were the second most prevalent in the world, therefore the development of new therapies is especially urgent.
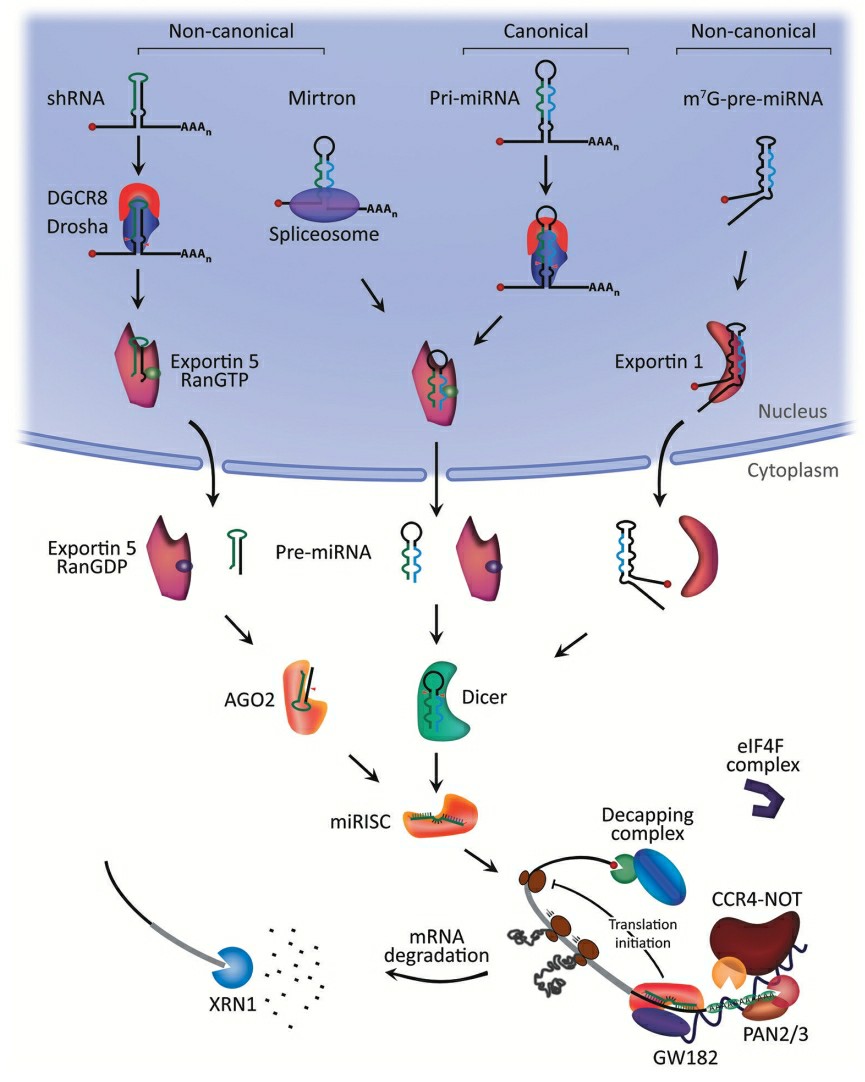 Fig. 1 MicroRNA biogenesis and mechanism of action1,6.
Fig. 1 MicroRNA biogenesis and mechanism of action1,6.
Delivery systems for miRNA
- Viral vector systems
Retroviridae is a family of seven genera, and members of these genera are used as viral vectors for the delivery of miRNAs. The vast majority of the studies involving this approach have focused on members of the gamma-retrovirus and lentivirus genera, including HIV-1. One of the disadvantages of using gamma-retroviral vectors to deliver miRNAs to non-dividing cells is that transduction efficiency is quite low. On the other hand, LVs can transduce non-dividing and slowly dividing cells and can therefore be used to deliver miRNAs to most tissues and organs including the central nervous system (CNS). Recombinant adeno-associated viruses (AAVs) are among the most successful viral vectors for delivering miRNAs. These vectors are the least pathogenic, have low host immunogenicity, target most cell types and tissues, have high transduction efficiency, and have a long-term expression potential. It has been shown that recombinant adenovirus infection of glioblastoma stem cells (GSCs) after direct intracranial injection is feasible.
- Non-viral delivery system
Lipid vesicles (liposomes) consist of one or more lipid layers (i.e., one or more concentric closed bilayers of phospholipids with their hydrophobic heads and hydrophilic tails) enclosing a water-soluble core. As a result, the use of liposomes for the delivery of water-soluble substances has become popular, and they are the most commonly used vector for the delivery of miRNAs. This is because of their appropriate size (approximately 100 nm), good biocompatibility, and easy preparation and application. Here, the research on the therapeutic effect of miR-7 in human glioblastoma in vivo. Peptides are also good vehicles for siRNA and have been reported as good vehicles for transporting siRNA. Cationic peptides, a family of peptides which are called cell-penetrating peptides (CPPs), are reported to be capable of delivering different kinds of macromolecules, including miRNAs, into tumor cells and other target cells.
Progress in Chemical Modifications of miRNAs for Improved Stability
The presence of a variety of chemical modifications to nucleobases, ribose sugar, or phosphate backbone may shield the negative charge of miRNAs and other nucleic acids. Modification in nucleobases, ribose sugar, or phosphate backbone of miRNAs and other nucleic acids may also contribute to the cell surface adherence. The adhesion of miRNAs to the cell surface subsequently aids in cellular uptake and bolster their stability as well. A type of nucleic acid modification commonly used is locked nucleic acid (LNA) bases, which include a methylene bridge. The methylene bridges constrain the flexibility of the ribose ring, so that the modified nucleotides have a locked conformation. The modified RNA nucleotides in the LNA-based therapeutics are more resistant to ribonucleases and show improved cellular uptake. Phosphorothioate modifications increase oligonucleotide stability while allowing it to enter cells by endosomal uptake via stabilin receptors on cell surfaces, specifically found on kidney cells. This approach was used for the specific delivery of the synthetic miR-21-anti-miR (RG-012/lademirsen/SAR339375) to the kidney in a clinical trial for Alport syndrome (NCT03373786, NCT02855268). Specific sequence-independent effects have been reported in relation to phosphorothioate-modified oligonucleotides.
miRNA Applications in Neurodegenerative Diseases
- Alzheimer's Disease
AD pathology research has recognized miR-135a-5p as an important factor. In mice with an AD phenotype, miR-135a-5p levels in excitatory hippocampal neurons are decreased. Reduced miR-135a-5p levels cause synaptic and memory dysfunction in a tau-dependent manner. MiR-135a-5p targets the protein BACE1 by binding to its 3' UTR, thereby affecting the amount of Aβ plaques, which are a common AD feature. Another study found that DAT patients have higher levels of miR-135a in their serum exosomes. The results from these studies indicate that miR-135a-5p could be a potential therapeutic target for AD.
- Parkinson's Disease
MiR-155 and miR-141 have been proposed as a biomarker and therapeutic target in PD. The two miRNAs regulate neuroinflammation and α-synuclein. α-synuclein has been found to be a protein responsible for the disease. For instance, it has been found that miR-155 mediates the neuroprotective effect of interferon-γ signaling in a mouse model of AD. Moreover, miR-141 regulates α-synuclein. This research proves that the two miRNAs can be used as a marker for early detection and progression of the disease and as a therapeutic target.
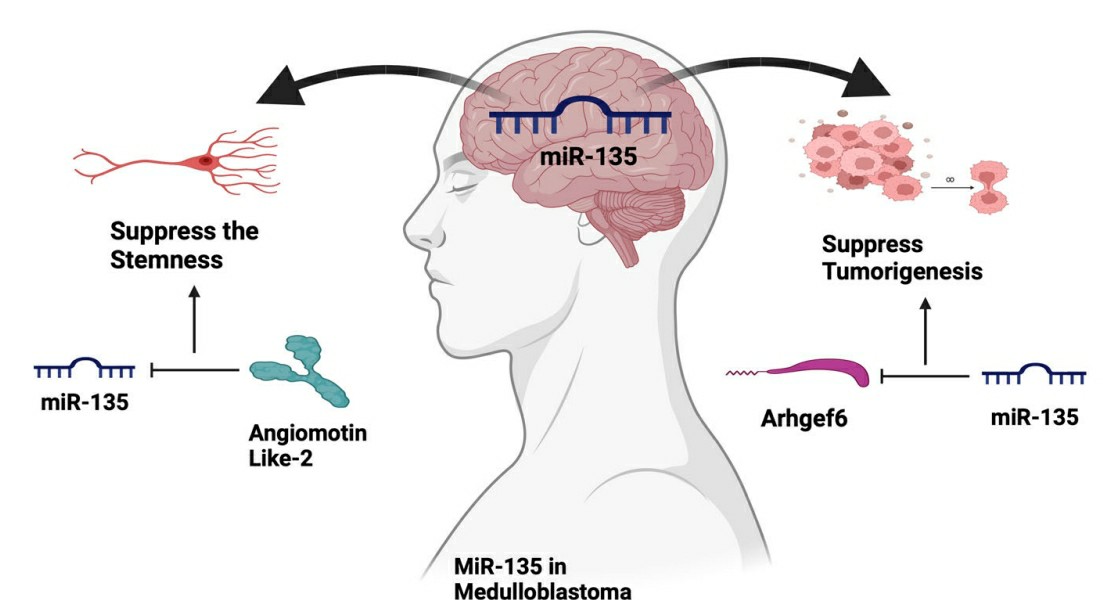 Fig. 2 The schematic represents the potential roles of miR-135 in medulloblastoma2,6.
Fig. 2 The schematic represents the potential roles of miR-135 in medulloblastoma2,6.
- Huntington's Disease
There is potential for MiR-124 to reduce the effects of HD. In HD mice, administration of miR-124 via exosomes resulted in reduced inflammation and improved motor performance. MiR-124 targeted inflammatory pathways and neuronal survival genes for a double benefit of reduced neuroinflammation and increased neuronal health. The use of exosomes as a delivery mechanism for miR-124 provided improved bioavailability and bioactivity within the brain, offering a promising therapy for HD.
- Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) develops through the degeneration of brain and spinal cord motor neurons. MiR-34a regulates the expression of X-linked inhibitor of apoptosis (XIAP) which plays a role in mediating oxidative stress-induced senescence, and Sirtuin 1 (SIRT1) which has a protective role against oxidative stress-induced apoptosis. SIRT1 is downregulated in PD. ALS patient-derived cell lines have a reduction of miR-34a which is rescued by treatment with enoxacin, a small-molecule drug stimulating microRNA biogenesis. Therefore, enoxacin and other microRNA biogenesis stimulating drugs could be used as potential treatments for ALS. Studies show that miR-155 levels are elevated in sporadic and familial ALS patients while brain-targeted inhibition of miR-155 in SOD1G93A mice leads to improved survival and extended disease duration. miR-338-3p targets some subunits of mitochondrial OXPHOS complexes and is also implicated in ALS in human patients and mouse models. ALS-related genes FUS, TARDBP and SOD1 block the Dicer enzyme from processing pre-miRNA when they are overexpressed in motor neurons of human ALS patients.
Future Directions
The miRNA-based therapy for neurodegenerative disease in the future will depend on the clearance of the hurdles in delivery of miRNAs into the CNS and the testing of the possibility of using miRNAs as biomarkers and therapeutic targets. There are many barriers to the miRNA therapy, including delivery of miRNAs across the blood-brain barrier (BBB). Many studies show that several approaches such as intrathecal infusion and ventricular osmotic pumps can deliver miRNAs across the BBB. Alternative nano-carriers such as lipid nanoparticles and polymeric micelles are being explored for increasing the stability, half-life and tumor targeting of miRNAs.
References
- O'Brien, Jacob, et al. "Overview of microRNA biogenesis, mechanisms of actions, and circulation." Frontiers in endocrinology 9 (2018): 402. https://doi.org/10.3389/fendo.2018.00402.
- Kapplingattu, Sarika V., Sujata Bhattacharya, and Yogita K. Adlakha. "MiRNAs as major players in brain health and disease: current knowledge and future perspectives." Cell Death Discovery 11.1 (2025): 7. https://doi.org/10.1038/s41420-024-02283-x.
- Seyhan, Attila A. "Trials and tribulations of MicroRNA therapeutics." International journal of molecular sciences 25.3 (2024): 1469. https://doi.org/10.3390/ijms25031469.
- Nappi, Francesco. "Non-coding RNA-targeted therapy: a state-of-the-art review." International Journal of Molecular Sciences 25.7 (2024): 3630. https://doi.org/10.3390/ijms25073630.
- Konovalova, Julia,et al. "Interplay between MicroRNAs and oxidative stress in neurodegenerative diseases." International journal of molecular sciences 20.23 (2019): 6055. https://doi.org/10.3390/ijms20236055.
- Distributed under Open Access license CC BY 4.0, without modification.
