Target-Ag™ Platform-Driven Antigen Engineering
- Hypoallergen Design and Modification: Utilizing our proprietary Target-Ag™ Platform, we design and modify hypoallergens by precisely removing or altering allergenic epitopes using genetic engineering or chemical modification.
- Safety Enhancement: Aims to maximize the reduction of IgE binding capacity, thereby significantly lowering the risk of allergic reactions during treatment, ensuring high safety for vaccine candidates.
- Potency Preservation and Enhancement: By preserving or optimizing T-cell activation epitopes, we ensure the modified antigen can still efficiently induce the immune system to shift from a Th2 response to a Th1/Treg immune tolerance response, guaranteeing high immunogenicity and efficacy.
Customized Vector and Adjuvant Screening
- Strategy Customization: We customize the screening of the most suitable adjuvants and delivery systems by matching the route of administration with the allergen type, ensuring the local immune effectiveness of the vaccine.
- Adjuvant Innovation and Optimization: Creative Biolabs screens TLR agonists (e.g., CpG-ODNs) and novel adjuvants/delivery systems to promote APC maturation, drive Th1 differentiation, and uses specific adjuvants/targeted delivery to boost Treg generation for long-term immune tolerance.
- In-Depth Delivery System Optimization: Our optimized delivery systems include nanoparticle design/formulation, enabling efficient APC targeting, antigen stability, controlled release, and sustained safe immune stimulation.
Immune Response Mechanism Evaluation
- Multi-Dimensional Preclinical Model Application: Comprehensive and in-depth in vivo evaluation of vaccine candidates' efficacy and safety is conducted in established preclinical animal models.
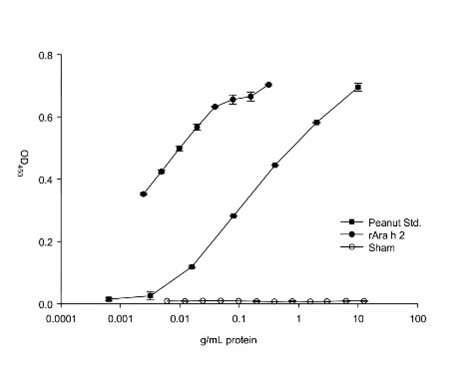
- Antibody Titer and Function Analysis: We evaluate sensitization reversal and allergic reaction inhibition by measuring serum total/specific IgE and quantitatively analyzing vaccine-induced IgG blocking antibody function.
- Immune Cytokine Profile Analysis: We utilize flow cytometry and ELISA techniques to monitor changes in Th1/Th2 cytokine and Treg marker profiles to validate the vaccine-induced immune deviation and tolerance mechanism.
- Functional Desensitization Verification: Through allergen challenge tests, we evaluate the practical function of the vaccine in inhibiting allergic symptoms in animals, directly verifying the desensitization efficacy.