Understanding the Molecular Mechanisms of Three RSV Vaccine Construction Strategies
Introduction: The Persistent Challenge of Respiratory Syncytial Virus (RSV)
Respiratory Syncytial Virus (RSV) stands as a major global respiratory pathogen, posing significant threats to public health worldwide. It is a leading cause of lower respiratory tract infections, particularly among infants, the elderly, and individuals with compromised immune systems. Epidemiologically, RSV spreads easily through respiratory droplets, with annual outbreaks often overwhelming healthcare systems during cold and flu seasons. Each year, it leads to millions of hospitalizations and a substantial number of deaths, especially in vulnerable populations.
Despite its profound impact, the clinical need for effective RSV prevention has long remained unmet. For decades, the development of RSV vaccines faced numerous challenges, ranging from safety concerns to difficulties in inducing long - lasting and potent immune responses. However, in recent years, there has been remarkable progress in RSV vaccine research and development. Several vaccine candidates have advanced to clinical stages, and some have even obtained regulatory approval, marking a new era in the fight against RSV. This article delves into the molecular mechanisms of three key RSV vaccine construction strategies, shedding light on their unique approaches and potential in addressing the global RSV burden.
The RSV F Protein: The Key Antigen and Its Critical Conformations
The RSV F protein is central to the virus's ability to infect cells, mediating the fusion of the viral and host cell membranes. This protein exists in two primary conformations, or shapes, that are crucial for vaccine design:
- Prefusion (preF) Conformation: This is the highly unstable form of the protein present before the fusion event. Crucially, the preF state exposes potent neutralizing epitopes (specifically designated as Ø and V sites) that are essential for eliciting strong protective antibodies. Stabilizing this preF conformation has been a primary focus in modern vaccine development.
- Postfusion (postF) Conformation: This is the stable, lower-energy form the protein adopts after mediating membrane fusion. Early vaccine efforts, which often used the less effective postF antigen, led to a lower neutralization efficacy.
Structural Stabilization: A Molecular Imperative
To harness the power of the preF state, molecular engineering techniques have been employed to lock the protein in this high-energy conformation. Strategies like introducing disulfide bonds, cavity-filling mutations, and specialized scanning techniques (such as Proline Scanning) have been instrumental in enhancing the antigen's immunogenicity and ensuring its stable presentation to the immune system.
Services you may interested in
Three Pillars of Modern RSV Vaccine Development
Modern strategies for RSV vaccine construction can be broadly categorized into three approaches, each leveraging the preF antigen but differing in delivery platform, production process, and immunological outcome.
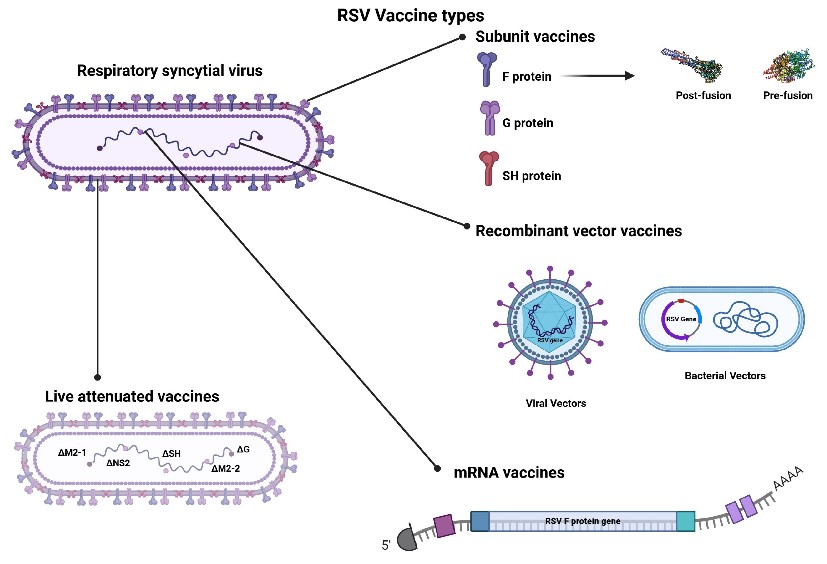 Fig.1 Three RSV vaccine types in Clinical Trials and Approved for Use.1,2
Fig.1 Three RSV vaccine types in Clinical Trials and Approved for Use.1,2
Strategy One: The Nucleic Acid Vaccine Platform
The nucleic acid (mRNA) platform represents a cutting-edge approach characterized by rapid development and high potency.
| Key Element | Mechanism/Content |
|---|---|
| Platform | Improved mRNA, often encapsulated in lipid nanoparticles (LNP), coding for a membrane-anchored, stabilized preF protein. |
| Antigen Design | Uses codon optimization and molecularly engineered preF stabilizing mutations (e.g., specific stabilizing sequences) to ensure high-quality protein production. |
| Immunological Outcome | The body's cells internally translate the mRNA into the preF protein. This in situ production induces a robust immune response, generating strong neutralizing IgG and a beneficial T-cell response (Th1-biased CD4⁺/CD8⁺). |
| Advantages | Offers rapid sequence iteration for new variant response, can be quickly scaled up for mass production, and avoids the need for viral culture. |
| Limitations | Requires extremely stringent cold chain conditions for storage and transport; safety monitoring is ongoing due to its relatively recent introduction for widespread human use. |
Key takeaway: This strategy leverages the host cell machinery for antigen production, resulting in an immune quality comparable to structurally stabilized preF protein vaccines.
Strategy Two: Recombinant Subunit Protein Vaccines
Subunit vaccines rely on producing the target protein antigen outside the body and then administering it directly, often with an immune-enhancing adjuvant.
| Key Element | Mechanism/Content |
|---|---|
| Platform | Full-length or truncated F protein (usually a trimer) expressed using established mammalian (e.g., CHO), insect (baculovirus), or bacterial (E. coli) systems. |
| Antigen Form | May use either a stabilized prefusion form (the modern, highly effective approach) or the postfusion form; requires the addition of adjuvants (like specified oil-in-water or aluminum-based types). |
| Immunological Outcome | Directly introduces the mature antigen, prompting B cells to generate neutralizing antibodies. Adjuvants are included to promote cellular immunity and help balance the T-helper cell response (Th1/Th2 balance). |
| Advantages | Benefits from mature, well-understood manufacturing processes. Cold chain requirements are less demanding than for mRNA vaccines. Some products are already fully approved by regulatory bodies. |
| Limitations | Production involves complex, high-purity protein purification, leading to relatively high manufacturing costs. If the older postfusion form is used, a significant drop in neutralization potency may occur. |
Key takeaway: This approach is valued for its established safety profile and suitability for vulnerable populations, such as pregnant individuals and infants, due to its mature production technology.
Strategy Three: Exclusive Prefusion F Protein Structural Design (Non-mRNA)
This strategy is focused purely on structural biology, meticulously designing and stabilizing the preF antigen, which can then be presented in various delivery systems (e.g., protein, nanoparticles, or viral vectors).
| Key Element | Mechanism/Content |
|---|---|
| Key Technology | Molecular stabilization techniques, such as Proline Scanning (introducing Proline mutations into key loops to maintain preF conformation). |
| Delivery Systems | Nanoparticles/Self-Assembly: Stabilized preF proteins are assembled into organized structures (trimers or multimers) to increase antigen density and focus the immune response on key neutralizing epitopes. Viral Vector Expression: Platforms (e.g., rAAV, Ad26-based) deliver the gene for preF in vivo, offering the additional benefit of inducing strong cellular immunity. |
| Immunological Outcome | Presentation of the critical preF epitopes (Ø, V) induces high-affinity neutralizing antibodies. The response also includes the activation of CD4⁺ Th1 cells, which are crucial for B-cell maturation and the quality of the antibody response. Nanoparticles and viral vectors can enhance delivery to lymph nodes, improving affinity maturation and the formation of memory B cells. |
| Progress | Clinical trials have demonstrated good safety and superior immunogenicity compared to older postfusion subunit vaccines. |
Key takeaway: This structurally guided method yields an exceptional immune response, comparable to the mRNA platform, by maximizing the exposure of the most potent neutralizing epitopes.
Comparative Analysis and Target Population Applicability
The selection of a vaccine platform often depends on target population, speed of response, and infrastructure.
| Comparison Metric | Ranking (Best to Least) |
|---|---|
| Research & Development Speed | mRNA > Subunit > Structured preF protein (requires complex engineering) |
| Production Cost | Subunit > Structured preF protein > mRNA (due to high LNP manufacturing costs) |
| Immune Quality (Neutralizing Potency) | Structured preF ≈ mRNA > Postfusion Subunit (due to low postfusion neutralization) |
Application Scenarios
- Elderly / Immunocompromised: Platforms generating high-titer neutralizing antibodies (mRNA and structured preF protein) are preferred to overcome potential immune senescence.
- Pregnant Individuals / Infants: Subunit vaccines, with their established safety and mature technology, are often the primary choice.
- Rapid Pandemic Response / New Variant Adaptation: The mRNA platform is uniquely positioned for quick sequence updates and deployment.
Future Directions and Research Hotspots
The ongoing evolution of RSV vaccine technology is focused on enhancing efficacy, breadth, and durability.
- Multivalent and Dual-Antigen Platforms: Developing vaccines that simultaneously target multiple pathogens (e.g., RSV A/B strains, or co-administration/co-coding with SARS-CoV-2 antigens) to offer 'one-shot' protection against multiple diseases.
- Next-Generation Adjuvants: Investigating novel immune-stimulators (e.g., activators for TLR7/8 or STING pathways) to push both mRNA and subunit vaccines towards a stronger, more desirable Th1-biased cellular response.
- Structure-Guided Optimization: Leveraging advanced structural biology tools (like Cryo-EM) and Artificial Intelligence (such as AlphaFold) to further fine-tune the stabilization and presentation of the preF antigen.
- Durability and Immune Memory: Deeper exploration of the mechanisms that sustain immune protection, particularly the formation and longevity of tissue-resident memory B and T cells, which is crucial for long-term protection, especially in older adults.
- Safety Monitoring: Continuous and vigilant assessment of potential risks, including Antibody-Dependent Enhancement (ADE) and Vaccine-Enhanced Respiratory Disease (VERD), particularly with new subunit or low-dose mRNA formulations.
A New Era of RSV Protection
The core principle driving the success of next-generation RSV vaccines is the precise molecular targeting of the prefusion F protein conformation. The three primary construction strategies—nucleic acid, recombinant subunit, and specialized preF structural design—are not mutually exclusive but rather complementary, offering a range of options with distinct advantages in antigen presentation, manufacturing, and immunological profile.
By integrating cutting-edge structural biology, advanced delivery technologies, and ongoing clinical data, the scientific community is now poised to deliver highly specific and durable protective solutions tailored to the diverse needs of different at-risk populations.
If you want to learn more about the norovirus vaccine, please refer to:
Respiratory Syncytial Virus F Protein in Next-Generation Vaccine Design
RSV Infection Life Cycle and Vaccine Target Discovery
The Translational Value of BRSV Models in Human RSV Vaccine
Deciphering the Molecular Mechanisms of Respiratory Syncytial Virus Infection
Browse our Norovirus Antigen Products
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Silva G S, Borges S G, Pozzebon B B, et al. Immune responses to respiratory syncytial virus vaccines: Advances and challenges[J]. Microorganisms, 2024, 12(11): 2305. https://doi.org/10.3390/microorganisms12112305
- Distributed under Open Access license CC BY 4.0, without modification.
Created October 2025
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


