Norovirus Vaccine Development – Where Are We Now in 2025
Norovirus stands as one of the most prevalent causes of acute gastroenteritis worldwide, affecting hundreds of millions of people annually. Its impact is particularly severe among vulnerable groups, including young children, older adults, and individuals with weakened immune systems. Symptoms such as severe vomiting, diarrhea, and abdominal pain often lead to hospitalizations—especially in elderly and pediatric populations—and can even be life-threatening in cases of dehydration or complications. Despite its global public health burden, no approved norovirus vaccine exists today, and treatment remains limited to supportive care, such as fluid replacement, and preventive measures like hand hygiene and sanitization. As we enter 2025, progress in norovirus vaccine research has accelerated, driven by innovative technologies and lessons from past viral outbreaks. This article explores the latest advancements in norovirus vaccine development, key challenges facing researchers, and the future outlook for bringing a safe, effective vaccine to market.
Current Progress in Norovirus Vaccine Research
After years of slow progress, norovirus vaccine development has gained momentum in recent years, with multiple candidates advancing through clinical trials and new platforms showing promise. Below is a breakdown of the most notable progress across key research areas.
Clinical trials are the backbone of vaccine development, and 2024–2025 has seen several norovirus vaccine candidates move into late-stage testing— a critical step toward approval.
mRNA Vaccine Platform
The success of mRNA technology in combating COVID-19 has opened new doors for norovirus vaccine research. One of the most significant milestones is the development of the first mRNA-based norovirus vaccine to enter Phase III clinical trials, a key step in evaluating its ability to prevent acute gastroenteritis. Launched in 2024, this trial aimed to assess the vaccine's safety and efficacy in thousands of participants across diverse regions, with a focus on high-risk groups. However, the trial was temporarily paused in late 2024 due to a safety review related to a rare neurological condition, a standard precaution in clinical research to ensure participant well-being. As of 2025, researchers are working to address the concerns raised, with plans to resume the trial once safety protocols are updated.
Beyond this candidate, mRNA technology offers unique advantages for norovirus: it can be rapidly modified to target new viral strains (critical for a highly variable virus like norovirus) and produced at scale. Yet challenges remain, including optimizing delivery systems to ensure the mRNA reaches target cells in the digestive tract (where norovirus primarily infects) and ensuring long-lasting immunity.
VLP (Virus-Like Particle) Vaccines
Virus-like particles (VLPs) have emerged as a leading platform for norovirus vaccines, thanks to their strong safety profile and ability to trigger robust immune responses. VLPs mimic the structure of norovirus but do not contain infectious genetic material, making them non-pathogenic while still prompting the body to produce protective antibodies.
Several VLP-based norovirus candidates are now in advanced clinical trials. One promising candidate has demonstrated strong immunogenicity in animal models—meaning it successfully stimulates the immune system to recognize norovirus—and is currently transitioning into Phase II or III trials as of 2025. Another VLP vaccine is being evaluated for its ability to protect against multiple norovirus strains, a key feature for addressing the virus's diversity.
What makes VLPs particularly appealing is their consistency: they can be manufactured using well-established biotech processes, reducing production timelines and costs. Early data also suggests they may be well-tolerated, with minimal side effects reported in early-phase trials.
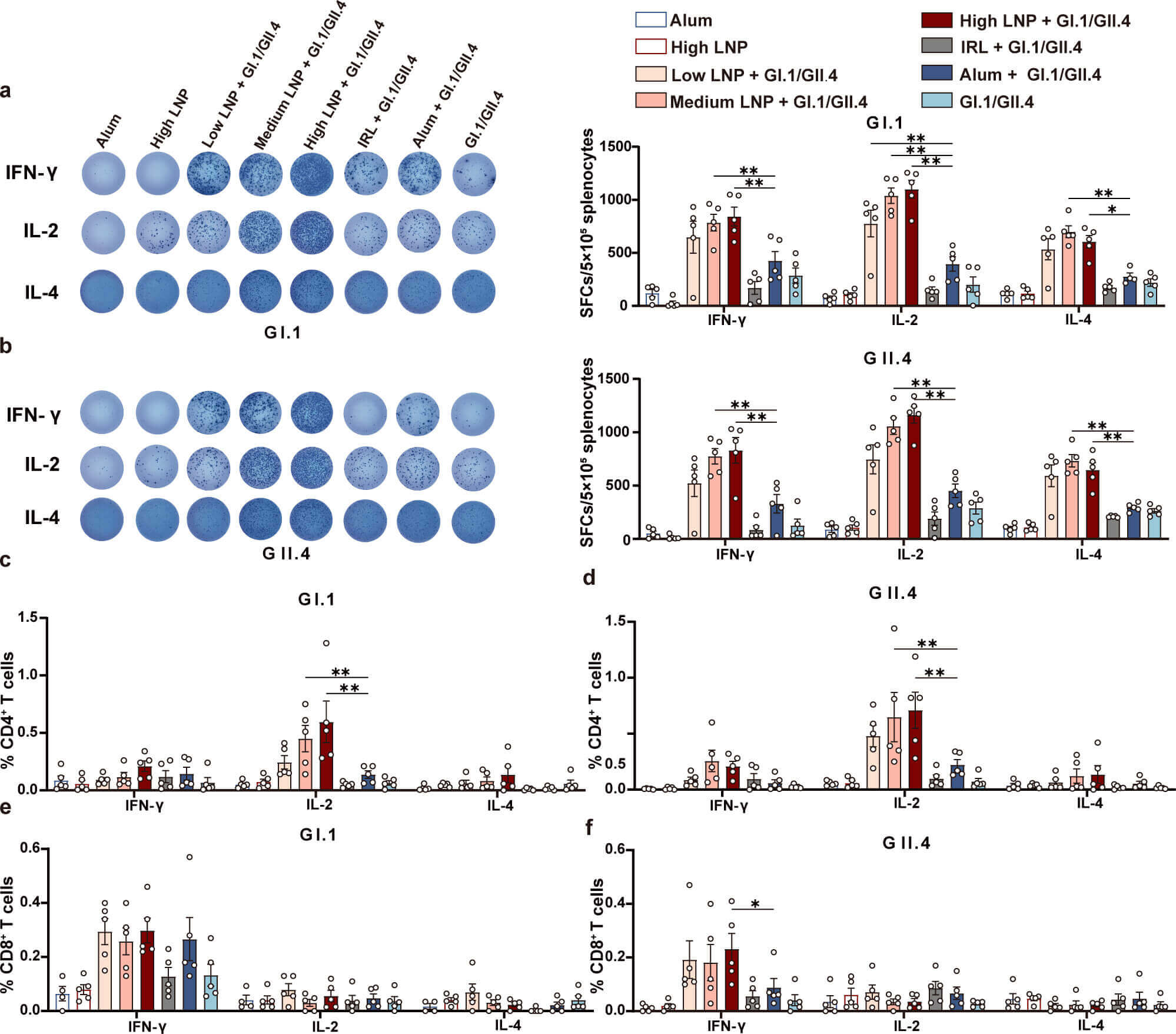 Fig.1 Memory T cell immune responses in norovirus VLP vaccine vaccinated mice.1,2
Fig.1 Memory T cell immune responses in norovirus VLP vaccine vaccinated mice.1,2
Oral Vaccines
Oral vaccines represent a game-changer for norovirus prevention, as they are easy to administer (no injections required), improve patient compliance, and can be distributed at scale—critical for reaching communities with limited healthcare access. A leading oral candidate, developed as a capsule, launched its Phase I clinical trial in 2024, with results expected to be finalized in 2025. If successful, the vaccine could advance to Phase III trials as early as 2026.
Oral vaccines work by targeting the digestive tract, where norovirus first invades the body, creating a localized immune response that blocks infection. However, developing oral vaccines requires overcoming challenges like ensuring the vaccine survives stomach acid and reaches the intestines intact— a hurdle researchers are addressing with novel formulation technologies.
Other Emerging Platforms
While mRNA and VLP vaccines lead the pack, other platforms are in early-stage development:
- Adenovirus vector vaccines: This technology has proven successful for other viruses (e.g., COVID-19, Ebola) but remains in the early stages for norovirus. Researchers are exploring how to use modified adenoviruses to deliver norovirus proteins and trigger immunity.
- DNA vaccines: DNA vaccines, which work by injecting genetic material that instructs cells to produce viral proteins, are still in the exploratory phase for norovirus. Their main advantage is stability (no need for cold storage), but they currently face challenges with low immunogenicity.
Table 1. Summary and Comparison Table of Norovirus Vaccine Technology Platforms
| Vaccine Technology Platform | Core Technical Features | Current R&D Progress (as of 2025) |
|---|---|---|
| mRNA Vaccine Platform | Draws on the successful experience of COVID-19 vaccines, can be rapidly modified to adapt to viral mutations, and can trigger targeted immune responses, but needs to address issues such as targeted delivery | The first mRNA norovirus vaccine entered Phase III clinical trials, which was paused in 2024 due to review regarding the Guillain-Barré syndrome incident |
| Virus-Like Particle (VLP) Vaccine | Does not contain viral infectious genetic material, featuring high safety and strong immunogenicity, and can be mass-produced through mature biotechnological processes | Multiple candidate vaccines are in the clinical trial stage; one of them has demonstrated good immunogenicity in animal models and is entering Phase II or III clinical trials; another one is simultaneously undergoing clinical trial evaluation |
| Oral Vaccine | Administered in the form of oral capsules, with good compliance and high convenience, suitable for large-scale population application, and needs to overcome the challenge of withstanding the acidic environment in the stomach | Phase I clinical trials have been launched, with completion expected in 2025; it may advance to Phase III clinical trials in 2026 |
| Adenovirus Vector Vaccine | Draws on the successful experience of developing vaccines for other viruses, and delivers viral antigens to trigger immune responses by modifying adenoviruses | Its application is still in the early R&D stage and has not entered mid-to-late stage clinical trials |
| DNA Vaccine | Induces immune responses by introducing viral genetic material to guide the body's cells to express viral proteins; has the advantage of storage stability, but its immunogenicity needs to be improved | Its application is still in the exploratory stage and has not entered key clinical trial stages |
Services you may interested in
In Vivo ADME & PK Study for Vaccine
Key Challenges and Future Outlook
Despite recent progress, norovirus vaccine development still faces significant obstacles. Addressing these challenges will be critical to bringing a vaccine to market in the coming years.
Ongoing Technical and Research Challenges
Norovirus's High Variability
Norovirus is genetically diverse, with dozens of genotypes and subtypes circulating globally. This variability means a vaccine that works against one strain may not protect against others—a major barrier to broad prevention. For example, the most common norovirus genotype (GII.4) evolves rapidly, with new variants emerging every few years. This requires vaccines to either target multiple strains simultaneously or be updated regularly, adding complexity to development and manufacturing.
Lack of Effective Animal Models and Cell Cultures
Studying norovirus in the lab is difficult due to the lack of reliable animal models and cell culture systems. Most animals (e.g., mice, rats) do not develop symptoms similar to human norovirus infection, making it hard to test vaccine efficacy before human trials. Similarly, norovirus does not replicate well in standard cell cultures, limiting researchers' ability to study how the virus infects cells and how vaccines can block it. While some progress has been made (e.g., using humanized mice or organoids), these models are expensive and not widely available, slowing down research.
Long-Term Safety and Efficacy Testing
Vaccines require rigorous long-term testing to ensure they are safe and provide lasting protection. For norovirus, this means tracking participants in clinical trials for years to assess:
- Immune persistence: Do vaccine-induced antibodies remain at protective levels over time?
- Long-term safety: Are there rare or delayed side effects that do not appear in short-term trials?
- Real-world efficacy: Does the vaccine work in diverse populations (e.g., children, older adults) and in settings where norovirus spreads easily (e.g., schools, nursing homes)?
These studies take time, and many current candidates have not yet completed long-term follow-up— a necessary step before regulatory approval.
Future Directions for Norovirus Vaccines
Despite these challenges, the future of norovirus vaccine development is promising, with several strategies poised to drive progress:
Multivalent and Broad-Spectrum Vaccines
To address norovirus's variability, researchers are focusing on multivalent vaccines (which target multiple known genotypes) and broad-spectrum vaccines (which protect against a wide range of strains, including future variants). Early data from VLP-based multivalent candidates suggests they can trigger immune responses against multiple norovirus genotypes, and mRNA technology could allow for rapid updates to include new strains. Broad-spectrum vaccines, meanwhile, aim to target conserved regions of the norovirus protein—parts of the virus that do not change across strains—offering long-term protection without frequent updates.
Advancements in Vaccine Technology
New technologies are helping overcome traditional barriers:
- mRNA-LNP delivery systems: Lipid nanoparticles (LNPs) improve mRNA stability and delivery to target cells. Researchers are optimizing LNPs to target the digestive tract, enhancing the effectiveness of mRNA vaccines against norovirus.
- VLP production optimization: New manufacturing techniques (e.g., yeast or insect cell cultures) are reducing the cost and time to produce VLP vaccines, making them more scalable.
- Oral formulation improvements: Novel coatings and encapsulation technologies are protecting oral vaccines from stomach acid, ensuring they reach the intestines and trigger an immune response.
Collaboration Across Sectors
Addressing norovirus's global burden will require collaboration between governments, healthcare organizations, and researchers. Initiatives like global norovirus surveillance networks are helping track circulating strains, guiding vaccine development. Public-private partnerships are also critical, as they provide funding for early-stage research and support large-scale clinical trials—especially in low- and middle-income countries, where norovirus outbreaks are often underreported.
Conclusion
As we stand in 2025, norovirus vaccine development is at a critical crossroads. While challenges like genetic variability and limited lab models remain, innovative platforms like mRNA and VLPs have brought us closer than ever to a safe, effective vaccine. The progress seen in clinical trials—including late-stage testing of mRNA and VLP candidates—offers hope that a norovirus vaccine could be approved in the next 3–5 years.
A successful norovirus vaccine would have a transformative impact on public health: reducing hospitalizations and deaths, preventing outbreaks in schools and nursing homes, and easing the burden on healthcare systems worldwide. For vulnerable populations like young children and older adults, it would provide much-needed protection against a virus that disproportionately affects them.
While there is still work to be done, the momentum in 2025 suggests that the era of norovirus vaccine prevention is within reach. As researchers continue to refine technologies, address challenges, and collaborate globally, we move one step closer to ending the global burden of norovirus-related illness.
If you want to learn more about the norovirus vaccine, please refer to:
Murine vs. Human Norovirus Models in Vaccine Research
Norovirus Immune Evasion and the Challenge of Durable Vaccine Protection
Virus-Like Particle Vaccines for Norovirus – A Promising Immunological Strategy
Browse our Norovirus Antigen Products
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Ford-Siltz, Lauren A., Kentaro Tohma, and Gabriel I. Parra. "Understanding the relationship between norovirus diversity and immunity." Gut microbes 13.1 (2021): 1900994. https://doi.org/10.1080/19490976.2021.1900994
- Distributed under Open Access license CC BY 4.0, without modification.
Created July 2025
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


