Murine vs. Human Norovirus Models in Vaccine Research
Introduction: The Global Burden and Research Imperative
Norovirus is a leading cause of acute gastroenteritis globally, causing 685 million annual infections and over $60 billion in yearly economic losses from healthcare costs and lost productivity. A key barrier to developing countermeasures has been the inability to culture human norovirus (HuNoV) in standard labs, limiting research on viral biology, host interactions, and preclinical testing. To address this, alternative models are essential, and this review focuses on the foundational murine norovirus (MNV) model and human-relevant systems. No single model suffices; synergies between diverse tools (from MNV to human organoids) drive progress by offering a holistic view of virus-host interactions.
The Foundational Role of Murine Norovirus (MNV) Models
A Gateway to Understanding Viral Biology
MNV, which infects mice, shares key biochemical/genetic traits with HuNoV (e.g., virion size, genome organization). Its greatest strength is tractability—it is the only norovirus reliably culturable in vitro (e.g., RAW 264.7 macrophages) and in vivo. This allowed scientists to study norovirus life cycles and host immunity before HuNoV systems existed, identifying viral tropism for macrophages/dendritic cells. This led to a pivotal hypothesis: HuNoV might also infect immune cells, challenging the long-held belief it only targets intestinal epithelial cells.
A Tractable Platform for Efficacy and Immunity Studies
MNV is vital for preclinical vaccine research, aiding studies of virus-host interactions, immunogenicity, and protective efficacy. Live MNV vaccination induces long-term immunity in mice; further research showed humoral immunity (antibodies) drives protection—serum from vaccinated mice protects others, while CD4+/CD8+ T-cell transfer does not. This guides vaccine development toward antibody-focused platforms (e.g., VLPs, mRNA). MNV also supports multivalent vaccines: multivalent VLP vaccines reduce viral loads post-homologous challenge and induce broad antibody responses against non-vaccine heterologous strains.
Acknowledging the Limitations
MNV does not perfectly mimic HuNoV infection. Differences exist in host receptors, immune mechanisms, and pathogenesis—MNV rarely causes severe gastroenteritis in mice, unlike HuNoV in humans. Thus, MNV is a foundational, not definitive, tool; its findings need validation in human-relevant systems.
Services you may interested in
Toxicology & Safety Assessment for Vaccine
In Vivo ADME & PK Study for Vaccine
The Breakthroughs in Human Norovirus (HuNoV) Modeling
Advanced In Vitro Systems: Human Intestinal Organoids
3D human intestinal organoids (HIEs/HIOs) from stem cells are a game-changer. They mimic native intestinal epithelium, supporting HuNoV replication and enabling direct study of HuNoV biology, host interactions, and drug/antibody screening. Recent advances (e.g., optimized commercial media for higher replication, genetically modified lines like J4FUT2 knock-in HIEs for broader strain susceptibility) enhance utility. For example, HuNoV replication in organoids is sensitive to interferons, challenging prior assumptions and identifying new therapeutic targets.
Small-Animal Models: Genetically Manipulable Systems
Immunocompromised (Rag-γc-deficient) mice are the first genetically manipulable small-animal model for HuNoV, supporting replication via immune deficiency (not human-engrafted cells). Their genetic tractability, cost-effectiveness, and scalability accelerate HuNoV biology research and antiviral evaluation.
Non-Murine In Vivo Models
Gnotobiotic piglets share human gastrointestinal traits and HBGA expression, replicating full HuNoV disease (e.g., diarrhea, shedding) and confirming HBGA's role in viral binding. Non-human primates (e.g., rhesus macaques) mimic human immune responses to HuNoV but rarely show diarrhea and face ethical/logistical constraints. Model selection depends on research goals—genetically manipulable mice for basic research, piglets for efficacy studies, and primates for late-stage immunological validation.
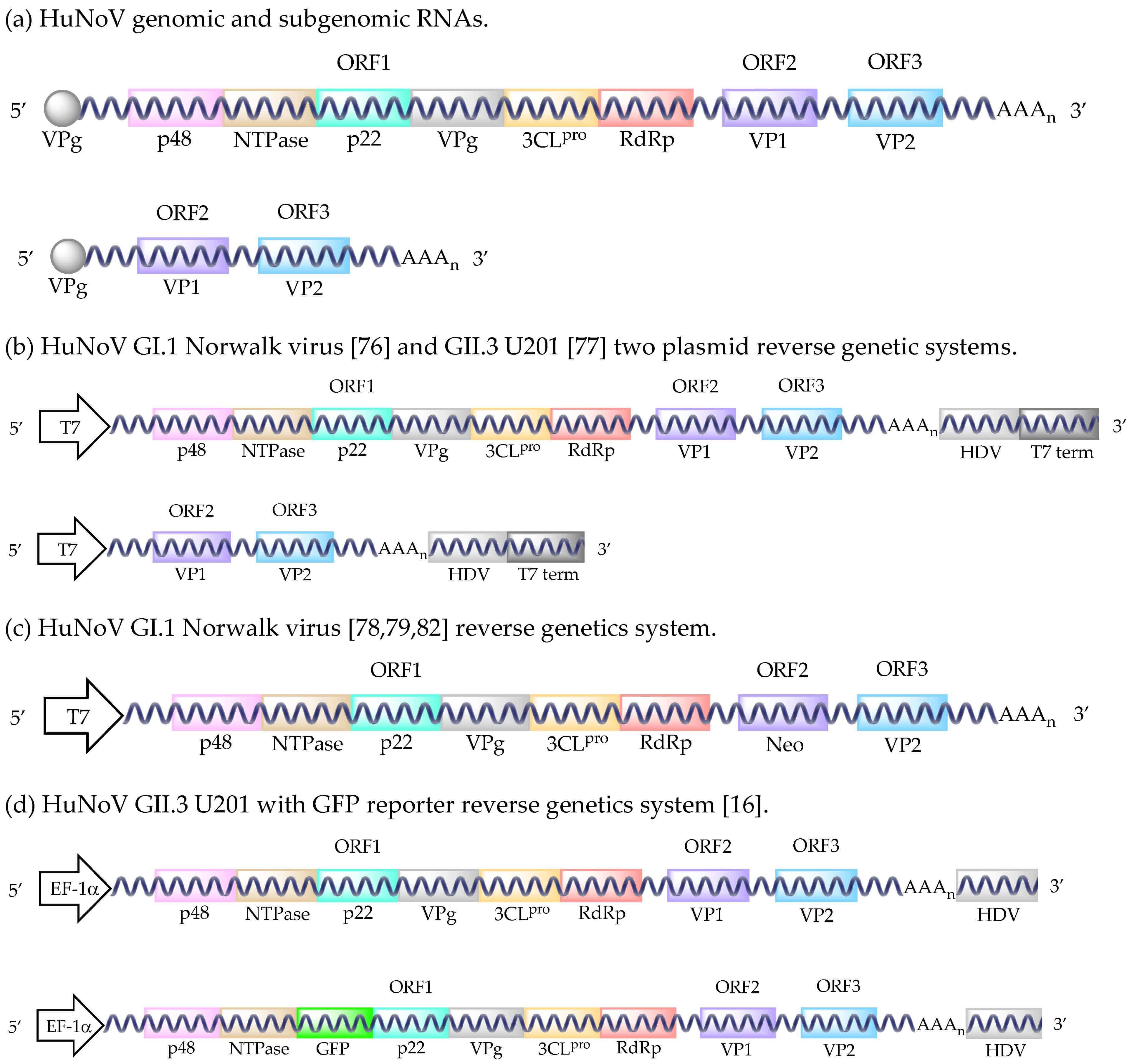 Fig.1 The diagram of the HuNoV reverse genetic system.1,2
Fig.1 The diagram of the HuNoV reverse genetic system.1,2
Table 1: Comparative Analysis of Norovirus Research Models
| Model | Key Advantages | Key Limitations | Primary Applications |
|---|---|---|---|
| Murine Norovirus (MNV) |
- Only norovirus routinely culturable in vitro/in vivo - Tractable for high-throughput studies - Insights into viral life cycle/immunity |
- Different pathogenesis/immune response vs. HuNoV - No severe gastroenteritis |
High-throughput screening, host-virus interaction studies |
| Human Intestinal Organoids (HIEs/HIOs) |
- Human-derived, mimics intestinal epithelium - Supports HuNoV replication - Genetically manipulable |
- Complex, expensive, not scalable - Low viral titers - Lacks full immune system |
HuNoV replication study, drug/antibody screening |
| Immunodeficient Mice |
- First genetically manipulable HuNoV small-animal model - Cost-effective |
- Requires immunocompromise - No full human disease |
Antiviral evaluation, in vivo replication research |
| Gnotobiotic Piglets |
- Recapitulates full human disease - Human-like GI traits/HBGA |
- High cost/logistical challenges - Not genetically tractable |
Vaccine efficacy, pathogenesis studies |
| Non-Human Primates |
- Closest to human immunity - Supports replication/adaptive responses |
- Ethical/cost constraints - No full clinical disease |
Late-stage vaccine validation, immunogenicity studies |
Synergistic Applications in Vaccine and Therapeutic Development
The Power of Genetic Engineering
Reverse genetics (e.g., plasmid-based systems) creates infectious HuNoV from cDNA, enabling precise genome manipulation (e.g., GFP insertion) to study viral life cycles.
Current Vaccine Development Landscape
HuNoV's genetic/antigenic diversity (rapid evolution, multiple genogroups/genotypes, GII.4 antigenic drift) complicates vaccines. Current candidates are multivalent (like influenza), targeting common genotypes. Advanced trials include multivalent VLP and mRNA vaccines (encoding VP1). Controlled human infection models (CHIMs) accelerate testing by exposing volunteers to HuNoV, providing accurate efficacy data faster than field trials. Broadly neutralizing antibodies targeting conserved viral regions also offer post-infection therapy for immunocompromised patients.
Table 2: Overview of Norovirus Vaccine and Therapeutic Platforms in Development
| Platform/Approach | Mechanism of Action | Development Status | Relevance to Key Challenges |
|---|---|---|---|
| Virus-Like Particle (VLP) Vaccines | VP1-based VLPs mimic virus to induce antibodies | Clinical/pre-clinical | Multivalent for antigenic diversity |
| mRNA-based Vaccines | mRNA encodes VP1, triggering immune response | Clinical/pre-clinical | Rapid updates for antigenic drift |
| Oral Tablet Vaccines | Oral administration induces mucosal immunity | Clinical/pre-clinical | Targets GI infection site |
| Antiviral Protease Inhibitors | Inhibit viral protease to stop replication | Clinical/pre-clinical | Strain-spanning efficacy |
| Monoclonal Antibody Therapy | Lab-made antibodies neutralize virus | Pre-clinical | Post-infection therapy for high-risk groups |
Norovirus research advances via a toolkit of models/technologies. From MNV's foundational insights to organoids' human relevance and piglets/primates' validation, each model fills gaps: MNV for high-throughput screening, organoids for human-specific studies, and larger animals for efficacy/immunity tests. Combined with genetic tools (reverse genetics), this approach defines norovirus illness, identifies therapeutic targets, and develops multivalent vaccines. Despite challenges, this adaptable framework offers optimism for effective countermeasures, marking a new era in infectious disease research where model synergies accelerate translation from discovery to clinical solutions.
If you want to learn more about the norovirus vaccine, please refer to:
Norovirus Immune Evasion and the Challenge of Durable Vaccine Protection
Norovirus Vaccine Development – Where Are We Now in 2025
Virus-Like Particle Vaccines for Norovirus – A Promising Immunological Strategy
Browse our Norovirus Antigen Products
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Todd, Kyle V., and Ralph A. Tripp. "Human norovirus: experimental models of infection." Viruses 11.2 (2019): 151. https://doi.org/10.3390/v11020151
- Distributed under Open Access license CC BY 4.0, without modification.
Created July 2025
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


