Norovirus Immune Evasion and the Challenge of Durable Vaccine Protection
Norovirus stands as a major global public health threat, imposing a substantial epidemiological burden. Each year, it claims over 1 million lives worldwide, with infants and the elderly being the most vulnerable groups. The urgent need for effective vaccines is undeniable, yet current vaccine development faces two critical bottlenecks: short - term protection duration and insufficient cross - protection against different norovirus strains. This article delves into the core mechanisms of norovirus immune evasion, the controversy surrounding the duration of natural infection immunity, the efficacy and limitations of existing vaccines, and explores breakthrough strategies and future directions to address these challenges.
Core Mechanisms of Norovirus Immune Evasion
Antigenic Drift Driven by Genetic Diversity
Genetic diversity is a key driver of norovirus immune evasion, primarily manifested through antigenic drift. Among various genotypes, the GII.4 genotype exhibits high - frequency mutations. These mutations alter the viral surface antigens, making it difficult for vaccines designed based on specific GII.4 strains to provide long - term and effective protection. Experimental challenge studies have provided clear evidence of the lack of cross - protection between different genotypes. When individuals are infected with one genotype of norovirus and then exposed to another genotype, they still face a high risk of reinfection, indicating that the immune response induced by one genotype has limited effect on other genotypes.
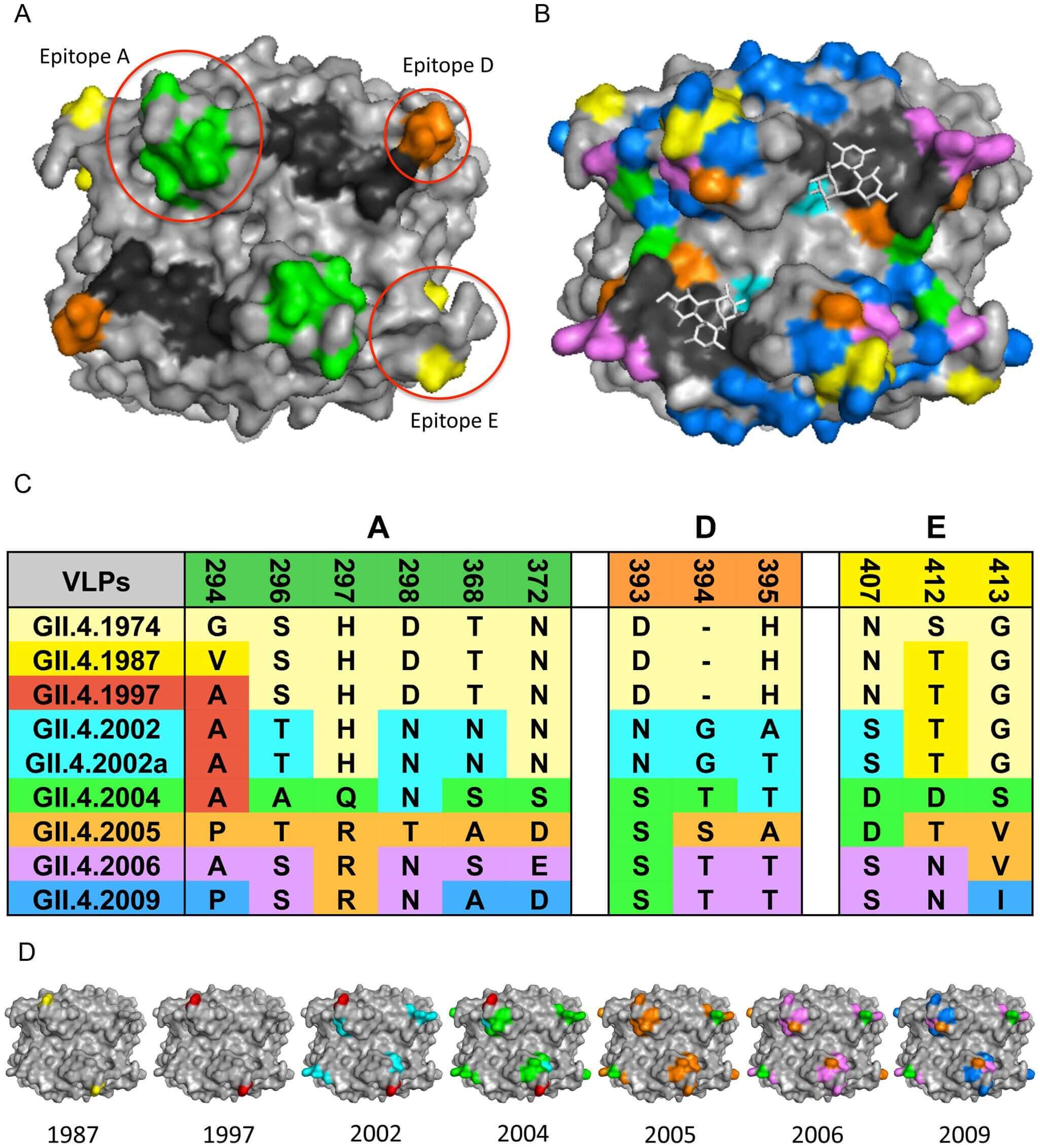 Fig.1 Tracking GII.4 norovirus genetic drift.1,2
Fig.1 Tracking GII.4 norovirus genetic drift.1,2
Restrictions Imposed by Host Genetic Factors
Host genetic factors also play a crucial role in norovirus infection and immune evasion, with HBGA (histo-blood group antigen) and the FUT2 gene being the key factors influencing susceptibility. Secretor + individuals, who express HBGA on the surface of intestinal epithelial cells, are at a high risk of norovirus infection. The reason is that norovirus can bind to HBGA, facilitating its entry into host cells and initiating infection. In contrast, non-secretor individuals, due to the absence of HBGA expression, show resistance to some norovirus strains. However, this resistance is limited, as certain norovirus variants can bypass this genetic restriction and still infect non-secretor individuals.
Limitations of Antibody Responses
The antibody response against norovirus has inherent limitations. There is a significant difference in the protective efficacy between mucosal IgA and serum IgG. Salivary IgA is associated with the reduction of clinical symptoms, such as alleviating vomiting and diarrhea. However, fecal IgA has weak protective effects against norovirus infection, failing to effectively prevent the virus from colonizing the intestinal tract. Moreover, the homologous antibody response induced by norovirus infection is strain - specific. This means that the antibodies produced after infection with one strain can only effectively neutralize that specific strain, but have little or no effect on other strains, further enhancing the virus's ability to evade the host immune system.
Services you may interested in
In Vivo ADME & PK Study for Vaccine
Controversy Over the Duration of Natural Infection Immunity
Evidence for Short - term Immunity
Challenge studies have provided evidence for short - term immunity against norovirus. The homologous protection period, i.e., the period during which individuals are protected from reinfection with the same strain after initial infection, is relatively short, ranging from 6 months to 2 years. Within 2 years of initial infection, if individuals are exposed to different norovirus strains, the risk of reinfection is high. This indicates that the immune memory induced by natural infection is not long - lasting enough to provide comprehensive protection against diverse strains.
Evidence for Long - term Immunity
In contrast, mathematical models based on community transmission data suggest a longer duration of immunity. These models estimate that the protective period may range from 4.1 to 9 years. However, there is a major controversy regarding this conclusion. Critics argue that in challenge studies, the dose of virus used is often much higher than the dose encountered in natural infections. This high - dose challenge may lead to an overestimation of the risk of reinfection and an underestimation of the actual protective effect of natural immunity, making the results of mathematical models and challenge studies difficult to reconcile.
Efficacy and Limitations of Existing Vaccines
Results of Clinical Vaccine Trials
Current clinical trials of norovirus vaccines have shown mixed results. The bivalent vaccine (GI.1/GII.4 VLP) is one of the widely studied vaccines. In healthy adults, this vaccine can reduce the incidence of clinical symptoms by 32% after virus challenge. However, the intensity of the antibody response induced by the vaccine is significantly lower than that induced by natural infection. For infants, a high - risk group for norovirus infection, the vaccine shows weak immunogenicity, meaning it fails to induce a strong enough immune response. Additionally, there is a lack of sufficient data on the side effects of the vaccine in infants, which limits its application in this population.
Challenges in Vaccine Persistence
The persistence of vaccine protection is another major challenge. Currently, the exact duration of protection provided by norovirus vaccines has not been clearly determined. Mathematical models predict that the protection period of vaccines may be shorter than that of natural infection. Furthermore, the antigen coverage of existing vaccines is insufficient. Newly emerging strains, such as the GII.4 Sydney strain, can escape the immune protection induced by existing vaccines, resulting in vaccine failure and continuous spread of the virus.
Breakthrough Strategies and Future Directions
Design of Broad - Spectrum Vaccines
To address the issues of insufficient cross - protection and antigenic drift, the design of broad - spectrum vaccines has become a research focus. One approach is to develop multivalent VLP (virus - like particle) vaccines that combine antigens from multiple genotypes (such as GI and GII). These vaccines have the potential to induce cross - reactive immune responses, providing protection against a wider range of norovirus strains. Another area of exploration is the association between T - cell responses and long - term protection. Studies suggest that T - cells may play a crucial role in maintaining long - term immune memory, and enhancing T - cell - mediated immune responses may be a key strategy to improve the durability of vaccine protection.
Translational Application Scenarios
In terms of translational applications, norovirus vaccines have specific value in certain scenarios. In closed environments such as cruise ships and military camps, where norovirus outbreaks are prone to occur due to dense populations and close contact, vaccines can provide short - term protection, reducing the risk and impact of outbreaks. For infant vaccination, there are important ethical considerations and a need for a careful assessment of the risk - benefit ratio. While infants are highly vulnerable to norovirus, the weak immunogenicity and unclear safety profile of current vaccines in this group require more in - depth research to ensure that the benefits of vaccination outweigh the potential risks.
Despite significant progress in norovirus research, several key knowledge gaps remain. Currently, there is a lack of reliable biomarkers for protective immunity. The identification of such biomarkers is crucial for evaluating the efficacy of vaccines, predicting the risk of infection, and guiding vaccine development. Additionally, the interactive mechanisms between genetic diversity, host factors, and immune memory are not fully understood. Further research in these areas is essential to develop more effective, long - lasting, and broad - spectrum norovirus vaccines, ultimately reducing the global disease burden caused by norovirus.
If you want to learn more about the norovirus vaccine, please refer to:
Murine vs. Human Norovirus Models in Vaccine Research
Norovirus Vaccine Development – Where Are We Now in 2025
Virus-Like Particle Vaccines for Norovirus – A Promising Immunological Strategy
Browse our Norovirus Antigen Products
Need a custom solution? If our off-the-shelf products aren't a perfect fit, we can create one for you. Contact us to design a product that precisely matches your experimental demands.
References
- Debbink, Kari, et al. "Norovirus immunity and the great escape." (2012): e1002921. https://doi.org/10.1371/journal.ppat.1002921
- Distributed under Open Access license CC BY 4.0, without modification.
Created July 2025
All of our products can only be used for research purposes. These vaccine ingredients CANNOT be used directly on humans or animals.


