γδ T cells are a small subset of peripheral blood T lymphocytes and can function as a promising tool for antitumor immunotherapy. Based on our advanced cell therapy platforms, Creative Biolabs provides one-stop CAR-γδ T cell development services. Our experienced scientists is capable of addressing the challenges of isolating and expanding γδ T cells.
γδ T cells can be divided into different types according to toTCRγ chains and TCRδ chains. Different γδ T cells have diversified distribution and functions. Based on the TCR structure, human γδ T cells can be divided into four main populations based on TCR δ chain expression (δ1, δ2, δ3, δ5). Vδ1 T cells are predominant in the thymus and peripheral tissues and recognize various stress-related antigens mostly uncharacterized. Vδ2 T cells constitute the majority of blood γδ T cells. In peripheral blood, T-cells expressing Vγ9Vδ2 TCR can account for up 95% of γδ T-cells and render between 1 and 10% of all blood T-cell. Vγ9Vδ2 T cells are promising candidates for cellular tumor immunotherapy.
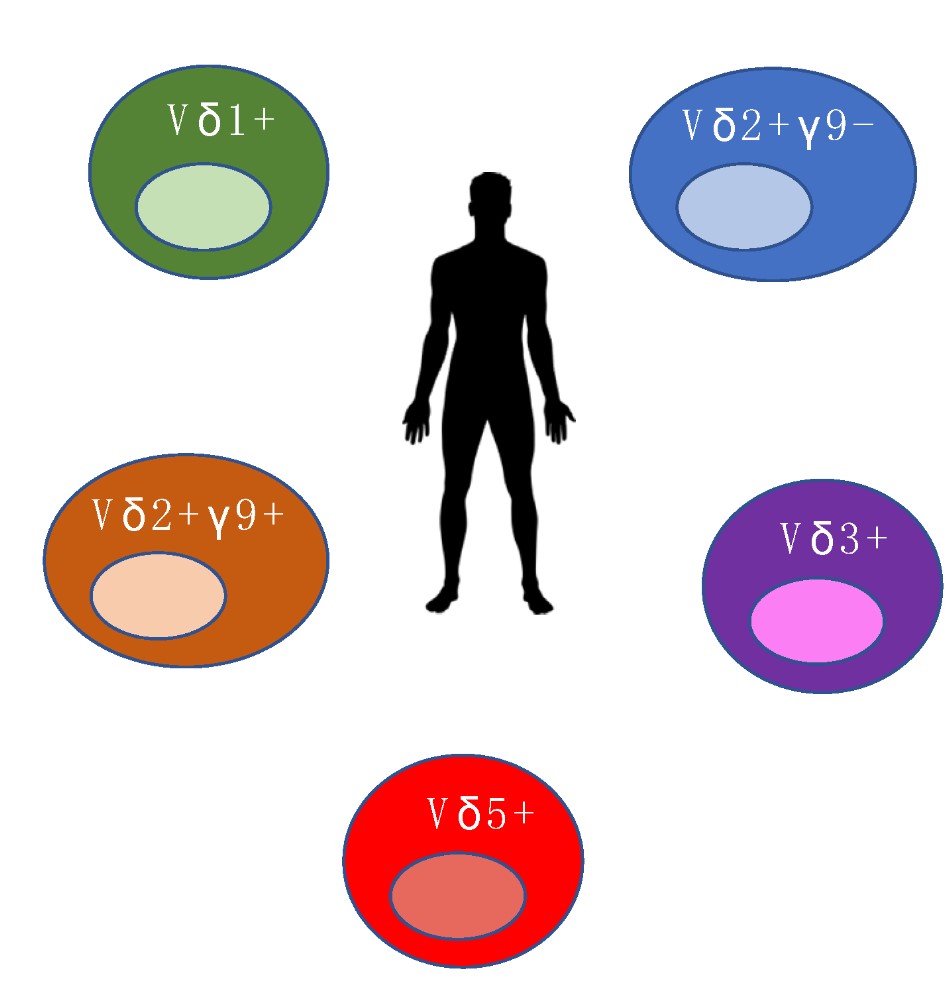 Fig.1 The different γδ T cell populations in human. (Xu, 2020)
Fig.1 The different γδ T cell populations in human. (Xu, 2020)
γδ T cells are unique unconventional T cells and are tissue-resident immune cells with inherent anti-tumor functions that do not rely on the expression of tumor-specific antigens and therefore provide a promising target for CAR cell therapy. However, these cells represent a small fraction (1-5%) of the peripheral T-cell pool and require activation and propagation to achieve clinical benefit. Typically, elaborate purification protocols such as FACS-based cell sorting or expansion in culture are needed to obtain enough γδ Tcells for subsequent studies. Now we provide an available approach to isolate and purify γδ T cells from peripheral blood mononuclear cells (PBMC).
There are many strategies to expand γδ T cells. Vδ2 T cells recognize in a TCR-dependent manner pyrophosphate intermediates of the eukaryotic and prokaryotic pathways of cholesterol synthesis, collectively termed phosphoantigens (pAg). The most potent pAg activators for Vδ2 T cells are isopentenyl pyrophosphate (IPP) and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP). In addition to pAg, nitrogen-containing amino-bisphosphonates (N-BP) are potent indirect activators of Vδ2 T cells. Most clinical ex vivo expansion strategies expand Vγ9Vδ2 T cells. The common protocol is the use of interleukin (IL)-2 plus a pAg or N-BP.
In addition, the feeder cell-based expansion method has also been investigated. The K562-derived aAPCs (K562VL6 (scFv-CD3-41BBL; scFv-CD28-IL15-RA)) provide a robust stimulation to all T cells. The expansion of the γδ T cells uses K562-derived aAPCs, following the isolation of γδ T cells. Vδ1+T cells from PBMC after depletion of αβ T cells can expand by culture with irradiated leukemia feeder cells and low levels of IL-2. In addition, it has been shown that plant-derived mitogens such as concanavalin-A (Con-A) can expand Vδ1 T cells.
An additional approach to expand Vδ1+and Vδ2+T cells is the use of an anti-γδ TCR antibody. Activated γδ T cells are prepared by incubating the resting γδ T cells with anti-γδTCR and anti-CD28 antibodies. These expanded γδ T cells showed an effective anti-lymphoma cytotoxicity and might be more suitable for adoptive immunotherapy of lymphoid malignancies.
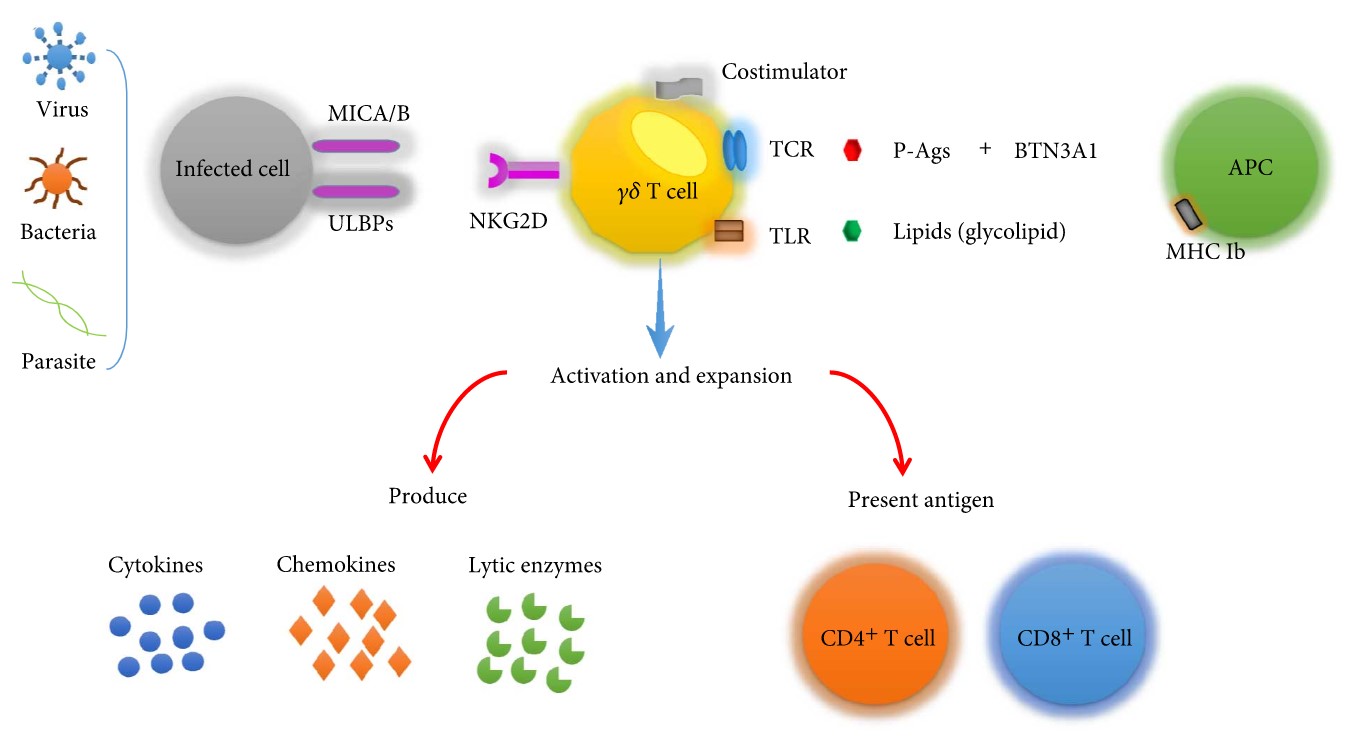 Fig.2 γδ T cells recognize antigens. (Zhao, 2018)
Fig.2 γδ T cells recognize antigens. (Zhao, 2018)
Adoptive immunotherapy with γδ T cells is a potential therapy for a variety of cancers. In vitro, γδ T cells can proliferate, be cytotoxic, or produce cytokines in response to various antigens. However, the broad application of γδ T cells for adoptive cell immunotherapy has been hindered by their low physiological frequency in the periphery, and the difficulty of ex vivo expansion. We are performing the expansion of γδ T cells using different strategies. In addition, we develop γδ T cells robust expansion kit to simply your study. The expansion of γδ T cells can reach 500~1000-fold and the purity of these cells is more than 80%.
Creative Biolabs focuses on studying the role of γδ T cell in the anti-tumor efficacy and developing CAR-γδ T cell-based therapies for cancer. If you are interested in our services, please feel free to contact us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION