Peripheral blood mononuclear cells (PBMCs) are critical components of the human immune system, comprising T cells, B cells, NK cells, and monocytes. They serve as a powerful foundation for cellular immunotherapies. Our platform leverages an innovative modified T central memory (mTCM) engineering approach to reprogram PBMCs into potent anti-tumor immune responders. By delivering tumor-associated antigens (TAAs) or synthetic peptide epitopes into PBMCs, and promoting their differentiation via cytokine cocktails, we generate dendritic cells (DCs) capable of cross-presenting multi-targeted antigens. These DCs induce the formation of tumor-targeting TCM cells with superior persistence, self-renewal capacity, and broad tumor recognition, setting a new paradigm for in vivo immunomodulation.
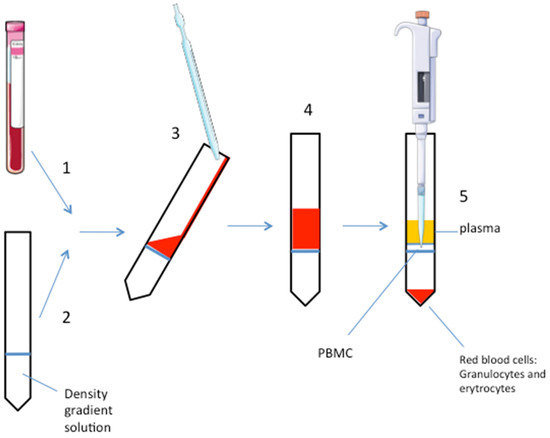 Fig.1 Separation process of PBMC.1
Fig.1 Separation process of PBMC.1
At Creative Biolabs, our in vivo PBMC engineering service encompasses:
We provide both standalone and integrated services, from construct design to in vivo evaluation, tailored to translational immunotherapy research.
Unlike traditional single-target approaches, our mTCM platform introduces multiple tumor-specific and pan-cancer epitopes into DCs, enabling a broader and more adaptive immune attack.
The engineered TCM population exhibits long-term persistence, maintaining surveillance and anti-tumor functions even post-treatment, reducing relapse risks.
From PBMC isolation to final in vivo validation, we offer a one-stop platform with expert guidance and quality assurance at each stage.
Our system minimizes reliance on cell freezing and recovery, allowing rapid turnaround for time-sensitive studies.
Our antigen delivery vectors are optimized to be non-integrative and non-replicative, ensuring biosafety for in vivo use.
To streamline your research process, we've designed a transparent and customizable workflow:
Our mTCM-based PBMC engineering approach has been referenced in multiple peer-reviewed studies and conference abstracts. Recent preclinical publications have demonstrated:
Q1: What types of tumors can mTCM technology target?
A1: Our system is adaptable to both hematologic malignancies and solid tumors by customizing the antigen cargo.
Q2: Are patient-derived PBMCs required?
A2: We support both autologous and allogeneic PBMC sources, depending on your study design and ethical approvals.
Q3: Can this service be integrated into CAR-T or TCR-T workflows?
A3: Yes. Our mTCM induction step can serve as a priming platform for downstream genetic modifications like CAR insertion.
At Creative Biolabs, we're committed to advancing in vivo immune modulation through innovation and partnership. Whether you are exploring preclinical efficacy, developing a novel T cell therapy, or seeking multi-target immunoengineering solutions, we are here to accelerate your success.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION