Instead of cutting the DNA, the deactivated Cas9 enzyme can be used to bind to specific gene sequences without causing any damage. This binding can be used to regulate gene expression or target specific genes for activation or repression. Creative Biolabs offers the allogeneic deactivated Cas9 gene-edited cell development service for precise and efficient gene editing in eukaryotic cells. By using deactivated Cas9 protein, we ensure the safety and accuracy of the editing process, minimize off-target effects, and maximize the success rate of the experiment. Our experienced team can customize cell lines to meet your specific research needs, whether for basic cell research or drug development projects.
As a form of genome editing, inactivated Cas9 gene editing uses a modified version of the Cas9 enzyme. Traditional Cas9 enzymes are responsible for the cleavage of DNA in target organisms, allowing the insertion or deletion of specific genes. However, in inactivated Cas9 gene editing, the Cas9 enzyme is altered so that it can no longer be cleaved in DNA. The inactivated Cas9 enzyme can be used to bind to specific gene sequences instead of cleaving DNA. This binding can be used to regulate gene expression or target specific genes for activation or repression.
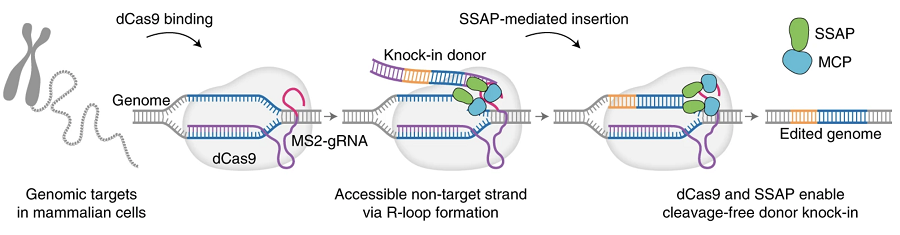 Fig.1 A schematic model of deactivated Cas9.1
Fig.1 A schematic model of deactivated Cas9.1
By using the deactivated form of the Cas9 enzyme, Creative Biolabs ensures that gene editing has no risk of off-target effects or accidental mutations. This technology is particularly useful for cell therapy, which can be used to correct genetic defects and treat various diseases. With extensive experience and a deep background in cell therapy, Creative Biolabs can help you easily develop custom cell lines for use in a wide range of disease research. Overall, Creative Biolabs' allogeneic inactivated Cas9 gene-edited cell development services provide a powerful and efficient tool for advancing genetic research and therapeutic interventions.
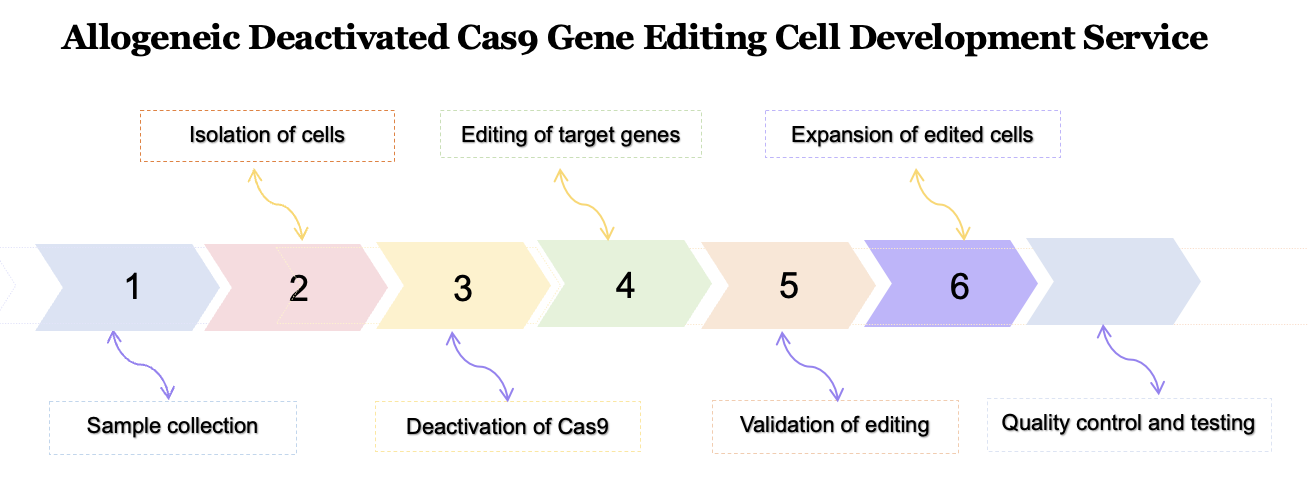
The first step could include blood samples, tissue samples, or any other relevant biological material.
The cells that will be edited using allogeneic deactivated Cas9 technology are isolated from the collected samples. This step could involve techniques such as cell sorting or centrifugation.
Once the cells are isolated, the Cas9 enzyme is deactivated using a process that eliminates its gene-editing capabilities.
Identify the target gene and make the necessary changes using the inactivated Cas9 enzyme. This step involves adding, deleting, or modifying specific sequences in the DNA.
After the gene editing has been performed, the edited cells are validated to ensure that the desired changes have been made successfully. This step includes techniques such as PCR or sequencing to confirm the presence of the edited sequence.
Once the editing has been validated, the edited cells are expanded in culture to generate a large enough population for further testing or use in therapies.
Throughout the process, quality control is necessary to ensure the safety and efficacy of the edited cells. This could involve tests for genetic stability, viability, or functionality of the edited cells.
Reduced risk of off-target effects
Allogeneic deactivated Cas9 gene editing can help minimize the risk of off-target effects, as it allows for precise and targeted editing of the CAR-T cells.
Improved safety profile
By deactivating the Cas9 enzyme after gene editing, the risk of unintended mutations or genomic instability in the CAR-T cells is reduced.
Scalability and manufacturing efficiency
Allogeneic CAR-T cell therapies can be mass-produced, providing a more scalable and cost-effective solution.
Enhanced potency and persistence
Deactivated Cas9 gene editing can be used to enhance the potency and persistence of CAR-T cells, improving their ability to target and kill cancer cells effectively over a longer period.
To learn more about our cutting-edge allogeneic deactivated Cas9 gene editing CAR-T cell development service and how it can revolutionize the field of cell therapy, please do not hesitate to contact us. Our team of experts is readily available to provide you with detailed information, answer any questions you may have, and explore how this innovative technology can benefit your research or clinical needs. Get in touch with us today to discover the endless possibilities that our service can offer.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION